PROPOSED RULEMAKING
MILK MARKETING BOARD
[ 7 PA. CODE CH. 144 ]
Electronic Methods for Testing Milk for Fat Content
[41 Pa.B. 2123]
[Saturday, April 23, 2011]The Milk Marketing Board (Board) proposes to amend 7 Pa. Code Chapter 144 (relating to electronic methods for testing milk for fat and component content) to read as set forth in Annex A.
Purpose of Proposed Rulemaking
The current regulations, adopted in 1987, specify, by manufacturer and model designation, which electronic testing instruments were approved for testing butterfat content of milk for purposes of payment to producers. Since that time, advances in testing technology and equipment, as well as changes in the way producers are paid for their milk, have made the current regulations obsolete and unworkable in practice. The purpose of this proposed rulemaking is to update the regulations to reflect these changes.
After lengthy consultation with industry and other governmental entities involved in testing milk, the Board is proposing comprehensive amendments to the regulations that will provide flexibility in adopting new technology, as well as accountability to insure that testing is well documented and performed in accordance with peer-reviewed science. The proposed rulemaking eliminates references to specific equipment and instead directs the regulated community to organizations that are recognized for establishing standards for equipment and methods for testing milk for the entire industry. The proposed rulemaking specifies how electronic milk testing equipment shall be maintained and calibrated while still providing language that is flexible enough to accommodate foreseeable changes in technology.
Summary of Proposed Rulemaking
When the existing regulations were adopted, producers' milk was priced based upon its butterfat (fat) content. Producers are now paid based upon ''multiple component pricing'' which uses butterfat, protein and other milk solids (such as lactose and minerals) to determine the price of milk. The proposed rulemaking adds the words ''and component'' to the Chapter 144 heading and replaces ''fat'' or ''butterfat'' with ''component'' throughout the regulations.
In § 144.1 (relating to electronic methods—general), proposed amendments to subsection (a) delete the requirement that the Board approve specific electronic instruments and reference methods in favor of language that allows methods to be used if they have been approved by one of several organizations that are recognized authorities in the field of electronic milk testing. Proposed subsection (b) reflects the fact that the Board will no longer require that specific instruments or testing procedures be preapproved by the Board. Subsection (c) is proposed to be deleted because the Board will no longer be designating, by manufacturer and model, which specific electronic testing instruments may be used. The recordkeeping requirements in this section that have not been rendered obsolete are proposed to be added to § 144.6 (relating to required records).
Proposed § 144.1a (relating to definitions) defines several terms that are used throughout the regulations.
Proposed amendments to § 144.2 (relating to certification and approval requirements) replace ''licensing'' with ''certification'' to be consistent with the terminology used in section 602 of the Milk Marketing Law (act) (31 P. S. § 700j-602). Proposed amendments to subsection (a) include government agencies and private institutions recognized for their authority in milk testing as additional approvers of electronic testing equipment. Subsections (b) and (d) are proposed to be deleted because the Board will no longer be approving specific test instruments, methods, locations or facilities nor licensing persons for specific reference methods or testing instruments.
Proposed amendments to § 144.3 (relating to laboratory facilities and supplies) delete language that was compatible with a narrow range of equipment or was superfluous because it was already required by another section. The reference to ''records'' has been removed because requirements for records maintenance and retention are proposed in § 144.6.
Proposed amendments to § 144.4 (relating to routine inspection and control) to remove from subsection (a) the specific directions on production and use of control samples and replace it with language that allows the Board to establish the standards for the production and use of control samples through Official General Orders as needed due to changes in technology. Subsection (b) is deleted because the daily performance checks will accomplish the same thing. Proposed subsection (b), formerly subsection (c), regarding daily performance contains the latest standards for accuracy checks and repeatability checks.
There are extensive proposed amendments to § 144.5 (relating to instrument calibration). The definition of ''calibration'' has been deleted and moved to proposed § 144.1a. References to the standard deviation calculation have been deleted in recognition of the fact that standard deviations are now calculated by computer. The proposed standards determine if an instrument is properly calibrated for different components. Subsection (c), regarding conditions requiring calibration, has also been amended to be consistent with the other amendments in this proposed rulemaking.
Section 144.6 has been expanded to include the recordkeeping requirements that are currently in other sections. The proposed amendments also allow for records to be maintained in electronic format, and further provide with more specificity exactly what shall be recorded and maintained for calibration and accuracy checks.
The current text of § 144.7 (relating to summary record required), currently titled chronological record required, is proposed to be deleted and replaced with proposed subsections (a) and (b). The current regulation requires a chronological record of butterfat tests using permanently bound or computer printed reports. The proposed text recognizes the industry trend toward use of commercial laboratories and computerized records. The proposed language more closely mirrors § 143.21 (relating to testing; notification of producer) by requiring two samples be tested in each half month rather than every 15 days.
Sections 144.8—144.10 (relating to date, sign, keep record 1 year; identify samples/tests; and identify test with patron's number) are proposed to be rescinded. These sections deal with recordkeeping requirements that are proposed to be added to § 144.6.
Section 144.11 (relating to two or more licensees) is also proposed to be rescinded because the practice of two or more licensees performing tests on a lot or group of samples is obsolete and no longer occurs in laboratories testing milk samples.
In proposed amendments to § 144.12 (relating to credit producers with actual component test), ''patron'' will be replaced by ''producer'' and ''fat'' will be replaced by ''component'' in both the section heading and the text. The amendment allows for rechecks to occur when the next sample is taken, rather than the current 72-hour requirement. The amendment also provides specific guidance as to which component measurements will lead to a recheck requirement.
Proposed amendments to § 144.13 (relating to availability of records) add cooperatives to the list of entities that must make records available to the Board. The amendment also changes the requirement that a written statement of test results be delivered to producers ''at each time a list is made'' to ''at least once each month,'' but allows these results to be communicated to producers by means of a verbal statement (such as an automated telephone dial-in or web access) rather than a written statement, if the producer agrees.
Proposed amendments to § 144.14 (relating to responsibility for violations) clarify that a certified tester at a laboratory or plant is responsible for a violation of the act or this chapter as well as the other parties listed in the existing regulations.
Statutory Authority
Section 307 of the act (31 P. S. § 700j-307) provides the Board with the authority to adopt and enforce regulations necessary or appropriate to carry out the act.
Public Hearing
On October 12, 2006, the Board, after due notice, conducted a public hearing to receive comments on a first draft of the proposed rulemaking. Among the attendees were representatives of the United States Department of Agriculture Milk Marketing Order #33, QC Laboratories, Dairylea Cooperative Inc./Dairy Marketing Services LLC, Independent Regulatory Review Commission (IRRC) and Board staff. As a result of the discussion and comments at that public hearing, the draft proposed rulemaking was revised and a second draft was circulated among the interested parties to receive further comment.
A second meeting was held on November 17, 2009, to discuss the second draft proposed rulemaking. Among the attendees at this meeting were representatives of the United States Department of Agriculture Milk Marketing Order #1, the United States Department of Agriculture Milk Marketing Order #33, Eastern Lab Services, QC Laboratories, Dairylea Cooperative Inc./Dairy Marketing Services LLC, Lancaster Dairy Herd Improvement Association and Board staff. The parties at this meeting suggested a few minor changes which were incorporated into this proposed rulemaking.
Fiscal Impact
The proposed rulemaking will not have fiscal impact on the regulated entities, the Commonwealth or its political subdivisions.
Paperwork Requirements
The proposed rulemaking will not require additional paperwork by the regulated entities, the Commonwealth or its political subdivisions.
Effective Date
The proposed rulemaking will become effective upon final-form publication in the Pennsylvania Bulletin.
Sunset Date
There is not a sunset date.
Regulatory Review
Under section 5(a) of the Regulatory Review Act (71 P. S. § 745.5(a)), on March 24, 2011, the Board submitted a copy of this proposed rulemaking and a copy of a Regulatory Analysis Form to IRRC and to the Chairpersons of the House and Senate Committees on Agriculture and Rural Affairs. A copy of this material is available to the public upon request.
Under section 5(g) of the Regulatory Review Act, IRRC may convey any comments, recommendations or objections to the proposed rulemaking within 30 days of the close of the public comment period. The comments, recommendations or objections must specify the regulatory review criteria which have not been met. The Regulatory Review Act specifies detailed procedures for review, prior to final publication of the rulemaking, by the Board, the General Assembly and the Governor of comments, recommendations or objections raised.
Public Comment
Interested persons are invited to submit written comments, suggestions or objections concerning the proposed rulemaking to the Chief Counsel, Milk Marketing Board, 2301 North Cameron Street, Harrisburg, PA 17110 within 30 days following publication in the Pennsylvania Bulletin.
RICHARD KRIEBEL,
ChairpersonFiscal Note: 47-15. No fiscal impact; (8) recommends adoption.
Annex A
TITLE 7. AGRICULTURE
PART VI. MILK MARKETING BOARD
CHAPTER 144. ELECTRONIC METHODS FOR TESTING MILK FOR FAT AND COMPONENT CONTENT § 144.1. Electronic methods—general.
(a) [The Board will approve electronic instruments and reference methods to determine the butterfat content of milk for payment purposes. Electronic instruments and reference methods submitted to the Board for approval shall be those recognized, approved and set forth in the latest edition of either Standard Methods for the Examination of Dairy Products, published by American Public Health Association, Washington, D.C. or Officials of the Association of Official Analytical Chemists (AOAC).
(b) A manufacturer requesting approval of an electronic butterfat testing instrument shall furnish to the Board a complete instrument operation and maintenance manual and further information as required. If, after approval of the electronic butterfat testing instrument by the Board, the manufacturer makes changes in the instrument, the testing procedure, the operating procedure or the maintenance instructions, the changes shall be submitted to the Board for approval prior to implementation of the change.
(c) Specific instructions for equipment and required reagents, testing techniques, equipment maintenance, related recordkeeping and other required procedures are as follows:
(1) Introduction. Some electronic fat testing instruments approved by the Board may be capable of determining the content of other components in milk, either by analysis or computation. Determination of milk components other than butterfat is outside the purview of this chapter and records of the determination are not required to be maintained. Factors which could affect the accuracy of the instrument for butterfat testing, such as certain maintenance procedures or total hours of instrument use, are subject to this chapter.
(2) Approved electronic fat testing instruments—manufacturer and model designation.
Milko Tester Mark II Milko Scan 104 (Instruments with automatic Milko Scan 203 diluent syringes only) Milko Scan 300 Milko Tester Mark III Milko Scan 133 Milko Tester Automatic Milko Scan 605 Milko Tester Mark III Industrial Multi-Spec. M (3) Manufacturer of instruments. The instruments in paragraph (2) are manufactured by A/S N. Foss Electric and Berwyn Instruments.
(4) Manufacturer requirements. The manufacturer shall submit complete instructions for the operation and maintenance of each model instrument for which approval is requested. Changes shall be submitted to the Board prior to distribution by the manufacturer.
(5) Required records. Records of the operation and maintenance of each electronic fat testing instrument shall be kept on forms prescribed and furnished by the Board, and shall contain the following information:
(i) Work sheet of calibration samples showing the following:
(A) Individual test results by electronic method and average result.
(B) Individual test results by reference method and average result.
(C) Description of adjustments made to electronic tester.
(D) Laboratory name and machine identification.
(E) Date, signature and number of technician.
(ii) Computation of standard deviation (SD) as follows:
(A) The results of individual samples by reference method and electronic method.
(B) The mathematical steps shown in computation of D and SD.
(C) The name of laboratory and machine identification.
(D) The date of computation, name and license number of technician.
(iii) Daily performance check showing the following:
(A) Name of laboratory and machine identification.
(B) Reference method used; sample identification, individual test results and average test.
(C) Electronic method used, time, sample identification, individual test results and average test results.
(D) Number of samples since rebuilding of homogenizer or other required maintenance procedures.
(E) Hours of machine operation on reporting date.
(F) Total hours of machine operation on instrument.
(G) Special maintenance. Copies of bills or service call reports for repairs or part replacements shall be kept with the maintenance records.]
Reference methods used to determine the component content of milk for payment purposes shall be those recognized or approved and set forth by the ICSMEDP in the latest edition of Standard Methods for the Examination of Dairy Products, published by American Public Health Association, Washington, DC, or by the AOAC in Official Methods of Analysis, published by AOAC International, Gaithersburg, Maryland. Only electronic instruments recognized by the USDA Dairy Division for the analysis of milk and milk components and capable of performance standards as referenced in § 144.4 (relating to routine inspection and control) shall be used to test milk for payment purposes in this Commonwealth.
(b) A manufacturer of an electronic testing instrument shall make available upon request to the Board a complete instrument operation and maintenance manual and further information as requested.
§ 144.1a. Definitions.
The following words and terms, when used in this chapter, have the following meanings, unless the context clearly indicates otherwise:
AOAC—The Association of Official Analytical Chemists.
Accuracy check—A test made at the beginning of each testing session and once per hour thereafter to determine the continued accuracy of the electronic testing apparatus.
Calibration—The adjustment of an electronic instrument so that the results for a given payment component meet the comparison criteria results of an AOAC or ICSMEDP approved reference method.
Certified tester—A Board certified technician as referenced in § 144.2 (relating to certification and approval requirements) operating electronic instruments or a person certified to perform specific reference methods for determining the components in raw milk, or both.
Control milk or control sample—Samples produced by a commercial laboratory or by the USDA Market Administrator's Office, or its successor agency, used to do the following:
(i) Determine the calibration of an electronic instrument.
(ii) Set the calibration of an electronic instrument.
Electronic method—A method for determining the components in raw milk using an electronic testing instrument.
ICSMEDP—The Intersociety Council on Standard Methods for the Examination of Dairy Products.
Milk component or component—A unique compound within milk whose relative mass within the milk may be used to determine the payment to producers. Component parts of milk include the following:
(i) Butterfat.
(ii) Protein.
(iii) Lactose.
(iv) Solids nonfat
(v) Other solids.
(vi) Total solids.
Reference method—A standard method using analytical chemistry or other approved techniques by which all other electronic methods of testing milk are compared for determining the components in milk.
Repeatability check—A test run at the beginning of each testing session to demonstrate the ability of a given electronic testing instrument or piece of equipment to meet the requirements for repeatability in § 144.4(b)(2) (relating to routine inspection and control).
USDA—The United States Department of Agriculture.
§ 144.2. [Licensing] Certification and approval requirements.
(a) No person may use an electronic instrument or method to test milk for [butterfat] component content for payment purposes unless the instrument and [test] method have been approved by the Board, ICSMEDP, AOAC or USDA Dairy Division, or their successor organizations.
(b) [No person may use an electronic instrument or method to test milk for butterfat content for payment purposes unless the specific test instrument and method, location and facilities have been approved by the Board.
(c)] No person may use or employ an electronic instrument or method to test milk for [butterfat] component content for payment purposes unless [licensed] certified by the Board under [Article VI] section 602 of the act (31 P. S. [§]§ 700j-602[—700j—608]).
[(d) A person testing milk by a reference method for the purpose of controlling the accuracy of an electronic test instrument or method shall be licensed for the method by the Board under Article VI of the act.]
§ 144.3. Laboratory facilities[,] and supplies[and records].
[Laboratories and other facilities using an electronic instrument or method to test milk for butterfat content for payment purposes shall have the following supplies, facilities and records available and in proper working order:
(1) An approved electronic testing instrument, required accessories and reagents, an instruction manual for operation of the instrument and an instrument maintenance record.
(2) A complete set of equipment and reagents for testing milk by the reference method approved for the purpose of controlling the accuracy of electronic testing instruments in use.
(3) A thermostatically controlled water bath, with recording thermometer having proper temperature distribution, set to maintain samples at 95°F to 100°F or at the temperature specified by the manufacturer of the electronic testing instrument.
(4) A power supply as specified by the manufacturer of the electronic testing instrument.
(5) A means of measuring pH.
(6) A supply of distilled or deionized water.
(7) Refrigeration at 33°—40°F for milk sample storage.
(8) A laboratory with adequate lighting facilities and adequate counter surface to accommodate essential equipment. The laboratory shall be free from disturbing drafts, dust, noise and vibrations.
(9) Hot and cold water, wash sinks and cleansing agents to clean equipment.
(10) An adequate waste and sewage system to dispose of milk, acid and wash water.
(11) Temperature and humidity controls and facilities as specified by the manufacturer of the electronic testing instrument.
(12) An approved preservative for milk samples as specified by the manufacturer of the electronic testing instrument.]
Laboratories and other facilities using an electronic instrument or method to test milk for component content for payment purposes must have the following supplies and facilities available and in proper working order:
(1) An approved electronic testing instrument, required accessories and reagents and an instruction manual for operation of the instrument.
(2) A thermostatically controlled water, or other manufacturer-prescribed medium, bath with recording thermometer having proper temperature distribution, set to maintain samples at the temperature specified by the manufacturer of the electronic testing instrument or other methods of obtaining the required temperature as specified by the instrument manufacturer and acceptable to the Board.
§ 144.4. Routine inspection and control.
(a) Preparation of control samples.
[(1) At least four control samples of natural milk of sufficient quantity shall be available to allow for performance checks and accuracy checks required by subsection (c).
(i) At least one control milk sample shall be a commingled sample of unhomogenized milk from a minimum of three herds or 100 cows, testing between 3.0% and 4.0% butterfat, and at least one control milk sample shall test between 4.5% and 6.0% butterfat. The calibration samples shall represent a variety of butterfat levels within the anticipated range of official samples to be tested.
(ii) An approved preservative shall be added at the required rate and mixed thoroughly if the control sample is to be used more than 24 hours after preparation. Churning shall be avoided. A sample shall be subdivided into subsamples of adequate size. The control sample shall be kept thoroughly mixed during subsampling.
(iii) A subsample of a control milk sample shall be tested in triplicate by the reference method for butterfat content. Individual determinations shall be read to at least the nearest 0.05% butterfat. The individual results and the average for a control milk sample shall be recorded.
(iv) The remaining control subsamples shall continue to be stored at 33°—40°F. until used. Subsamples more than 10 days old shall be discarded.
(v) Prior to the expiration date of the subsamples or use of the last subsample of the control milk sample, whichever comes first, preparation of a new set of control milk samples shall be completed.
(2) Standard mixtures approved by the Board may be used in lieu of the control milk samples. The mixtures shall be stored, tempered and tested in the manner prescribed by the Board.
(b) Instrument inspection prior to daily use. An electronic butterfat testing instrument shall be inspected prior to each day's use in accordance with the manufacturer's instructions. Deficiencies found during that inspection shall first be recorded in the instrument maintenance record and appropriate repairs or adjustments shall be made before the instrument is used to test milk.
(c) Daily performance.
(1) Accuracy check. Each day before routine testing begins, at least one subsample of control milk shall be tested in triplicate using an electronic instrument. The operator shall read the test to 0.01%. The first reading shall be disregarded. If the difference between the average of the second and third reading obtained from the electronic instrument and the average of the result obtained by the three reference methods is 0.1% butterfat or less, test three more samples of new control milk. If the difference of the additional samples exceeds 0.1% butterfat, the operator shall discontinue operation of the machine, determine the reason for the difference and correct the deficiencies before resuming operation. An accuracy check shall be performed at least once an hour during the time the electronic instrument is in operation.
(2) Repeatability check. Each day before routine testing begins, ten consecutive readings on a single well-mixed commingled sample of milk shall be made and recorded as a permanent record. The standard deviation of the results shall be less than 0.03% butterfat. If the standard deviation is 0.03% or greater, discontinue operation of the machine until the cause is determined and corrected. The standard deviation may be assumed to be acceptable if the range of the ten readings is .07% or less. Calculations of standard deviation are described in § 144.5 (relating to instrument calibration).]
Control samples shall be prepared in accordance with methods established by the Board through an Official General Order.
(b) Daily performance.
(1) Accuracy check. Each day before routine testing begins, at least once each hour during the course of the testing session, and when the testing session ends, at least one subsample of control milk shall be tested using the electronic instrument. The certified tester shall read the test to 0.01%. The result difference obtained by the reference method must be 0.05 or less than the known reference test sample result. If the difference of the samples exceeds 0.05, the certified tester shall discontinue operation of the instrument, determine the reason for the difference and correct the deficiencies before resuming operation.
(2) Repeatability check. Each day before routine testing begins, ten consecutive readings on a single well-mixed sample of milk that has not been homogenized shall be made and recorded as a permanent record. If more than ten consecutive readings are taken, the certified tester shall use the last ten results. The repeatability check is acceptable if the range of the ten readings is 0.04 or less.
§ 144.5. Instrument calibration.
(a) [Definitions. Calibration means adjustment of the settings on the instrument so that the butterfat test readings obtained from the instrument match the butterfat test result obtained by using the reference method approved by the Board.
(b) Calculation of calibration results.
(1) A machine shall be considered to be calibrated properly when the average difference between the machine results and the reference method results, called D, and the standard deviation of difference between methods, called SD, are less than the values described in Table 1. At least 20 samples shall be tested.
Table 1. Maximum Allowable Difference (D) and Standard Deviation of Differences (SD) Between the Electronic Tester and the Reference Method
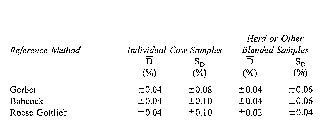
(2) The average of the results obtained by the low testing samples—3.0% to 4.0%—by the electronic method shall be compared to the average of the results obtained on the same samples by the reference method. If the difference is 0.02% butterfat or less, the calibration may be continued. If the difference is greater than 0.02% butterfat, the machine shall be adjusted and the samples retested by the adjusted electronic method until the difference is 0.02% butterfat or less.
(3) The average of the results obtained on the high testing samples—4.5% to 6.0%—by the electronic method shall be compared to the average of the results obtained on the same samples by the reference method. If the difference is 0.04% butterfat or less, the calibration procedure may be continued. If the difference is greater than 0.04% butterfat, the machine shall be adjusted and the samples retested by the electronic method until the difference is 0.04% butterfat or less.
(4) The criteria listed in paragraphs (2) and (3) shall be met simultaneously.
(5) The average difference between method, D, shall be calculated as the difference between the average of the electronic tester method on calibration samples and the average of the reference method on calibration samples. D, shall be considered as the mathematical equivalent of the following formula:
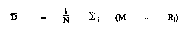
Where N = Number of samples tested
Mi = Average of electronic tester results on the ith sample
Ri = Average of reference method results on the ith sample, referred to as the ''true value''
(6) The standard deviation of difference, SD of calibration samples shall be calculated by a mathematical equivalent of the following formula:
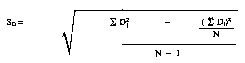
Di = Difference between the average of electronic tester results of the ith sample and the average of the reference method results for the ith sample.
(7) An example of the calculations required in paragraphs (5) and (6) is provided as follows:
Table 2
Sample Work Sheet for Determining
Standard Deviation
Column No. 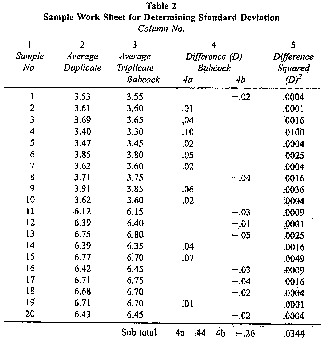
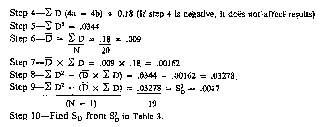
(8) The data in Table 3 provides sufficiently accurate estimate of SD.
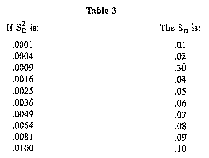
(i) In the example in Table 3, S2D was 0.0017, so the SD would fall between 0.04 and 0.05. To estimate that it was 0.04 is sufficient. If the value for S2D exceeds .0036 on blended milk or .01 on an individual cow's milk, the instrument shall be recalibrated.
(ii) The average difference (D) in the example is 0.009 and the standard deviation (SD) is 0.04, so the instrument is in proper calibration because these values are less than the values shown in Table 1 for the Babcock method for individual cow samples.
(iii) If either the mean difference or the standard deviation of difference, determined as outlined, exceed the values shown in Table 3, the instrument shall be adjusted as provided in subsection (b), and the calibration procedure repeated by retesting the same samples with the instrument.
(c) Conditions requiring calibration.
(1) The instrument shall be calibrated when initially installed.
(2) The instrument shall be calibrated when the performance check fails or the accuracy check fails.
(3) The instrument shall be calibrated if a part which may affect proper operation of the instrument is replaced, rebuilt or adjusted.
(4) The instrument shall be calibrated upon the occurrence of the specific circumstances which require calibration for that instrument, as set forth in this section.]
Calculation of calibration results. An instrument shall be considered to be calibrated properly when the average difference between the instrument results for butterfat and protein and the reference method results for at least ten different control samples, called mean average, is +/−0.04 and the standard deviation of the difference between the instrument and reference methods, called standard deviation, are 0.04 or less. For all solids the mean average is +/−0.09 and the standard deviation of the differences between the instrument and reference methods are 0.12 or less for those same ten samples.
(b) Conditions requiring calibration.
(1) The instrument shall be calibrated when initially installed.
(2) The instrument shall be calibrated when the accuracy check is confirmed to have failed.
(3) The instrument shall be calibrated if a part which may affect proper operation of the instrument is replaced, rebuilt or adjusted.
(4) The instrument shall be calibrated upon the occurrence of the specific circumstances which require calibration for that instrument, as determined by the manufacturer.
§ 144.6. Required records.
(a) [Records of butterfat tests shall conform to section 602 of the act (31 P. S. § 700j-602).
(b) Records of calibrations, performance checks, D and SD computations and other instrument use shall be maintained for 1 year under § 144.1 (relating to electronic methods—general).
(c) An instrument record shall be maintained for each test instrument in use under § 144.1.]
The certified tester and testing facility or laboratory shall maintain the records required under this section for at least 1 year. Records may be maintained in paper or electronic formats. Records must denote the record date and the name and license number of the certified tester who created or maintained the records.
(b) Records of calibrations, accuracy checks, mean average and standard deviation computations and other instrument use.
(c) Records of the operation and maintenance of each electronic testing instrument and records of test results by electronic method.
(d) Certified testers shall record standard deviation of the calibration verification as follows:
(i) The results of individual samples by reference method (average only for reference method) and electronic method.
(ii) The date of computation, name and license number of certified tester.
(e) Certified testers operating electronic testing equipment shall perform a daily accuracy check and record the following:
(i) Reference method used, sample identification, individual test results and average test.
(ii) Electronic method used, time, sample identification, individual test results and average test results.
§ 144.7. [Chronological] Summary record required.
[A record of butterfat tests shall be kept in chronological order either in a permanently bound record or in computer printed reports made at least once every 15 days. The record shall contain the farm sampling date, the lab testing date and the test result for each sample. The record shall be known as the original record or laboratory record. If bottles are numbered, the samples in each set shall be arranged for testing in numerical order, so that they may be reported in the same numerical order in the record book or printed report.]
(a) The certified tester and the testing facility or laboratory shall compile summary records of component tests performed for producers for the first and second half of each month containing results for at least two evenly spaced representative samples in each half month for each producer. The record must contain the farm sampling date, the laboratory testing date, the laboratory or testing site, the tester identification, the producer identification and the test result for each sample. The record shall be known as the original record or laboratory record and shall be maintained by the tester for at least 1 year. If the tests are performed by a milk dealer licensed by the Board, the milk dealer shall maintain the records of the component content of producers' milk samples for at least 1 year.
(b) If tests are performed in a commercial laboratory which is not an integral part of the milk plant where the samples were delivered, the licensed dealer or plant shall make available to the Board a copy of the final laboratory records of the component tests in computerized or written form for at least 1 year.
§ 144.8. [Date, sign, keep record 1 year] (Reserved).
[(a) Original records containing information with respect to the fat content of a producer's sample, whether the record is for 1 day or for more than 1 day, shall be dated and subscribed to by the person making the determination or by the technician or supervisor responsible for testing during the testing period for which the entry is made, and preserved for at least 1 year, regardless of the fact that the milk dealer may copy the record for the purpose of making a more permanent record for personal use.
(b) If tests are performed in a commercial laboratory which is not an integral part of the milk plant where the samples were taken, a carbon copy of the original laboratory records of the fat tests shall be prepared for transmittal to the plant where the samples were taken for filing purposes for at least 1 year.]
§ 144.9. [Identify samples/tests] (Reserved).
[If fat tests for different sets of samples or for samples representing different periods of time or different days are recorded on a single page in the original record book, ample space shall be used for the correct, clear and legible identity of a sample or sets of samples, to be followed immediately on the same page by the record of the fat tests thereon. The name and license number of the tester who made the tests or the technician or supervisor responsible for testing when the tests are made shall appear immediately following the fat-test record on a set of samples requiring a separate identity. The identification of a set of samples shall show the dates the fresh samples were taken or the limiting dates of the period which the composite samples represent. If there is more than one sample in a set, a sample as received at the laboratory shall be identified to distinguish it from others in the set. Show other available pertinent information to identify and characterize the sample.]
§ 144.10. [Identify test with patron's number] (Reserved).
[The percentage of milk fat found in the patron's sample shall be recorded opposite the distinctive number or mark assigned to a patron. Entries in the laboratory record book shall be made with an indelible pencil, with a pen and permanent ink or by a computer printed line of letters and numbers. If it is necessary to correct errors, corrections shall be made by drawing a line across the incorrect figure and placing the correct value nearby on the same line, or by adding an additional computer printed line clearly signifying the correcting information.]
§ 144.11. [Two or more licensees] (Reserved).
[If two or more licensees are performing tests on a lot of samples, each licensee shall work independently of the other to the extent of preparing a selected group of samples, including the measurement of the fat columns, and recording the results of the tests in the laboratory record book. The testing does not necessarily prohibit the common use of apparatus by the different licensees successively or simultaneously, such as the joint operation of the centrifuge, the joint use of the tempering bath, the use of the same balance and weights and the like. If two or more licensees are testing, the records as reported in the laboratory record book shall indicate the tests made by each licensee-name and license number subscribed to each series of tests and the completed tests shall be subscribed to—name and license number—by the responsible licensee in charge of the laboratory at the time the tests are made.]
§ 144.12. Credit [patrons] producers with actual [fat] component test.
(a) No [patrons] individual producers delivering milk or cream, or both, to a milk or cream-receiving or purchasing plant, where the milk or cream is purchased on the basis of the milk [fat] components contained therein, may be credited with a greater or lesser percentage or average percentage of milk [fat] components than is actually contained in the milk or cream delivered.
(b) No report on a test to determine the milk [fat] component content of milk or cream may be of a greater or lesser percentage of milk [fat] components than is actually contained in the milk or cream from which the sample was taken. [In order to] To be a basis of payment to [the patron] an individual producer, a recheck of a [patron's] producer's milk [fat] component test shall be made from [a] the next available sample taken [no later than 72 hours] after the original test. Rechecks of a producer's milk component test shall be made when the butterfat varies 0.5% or more or the protein varies 0.3% or more from the most recent test.
§ 144.13. Availability of records.
Laboratory, cooperative or plant records shall be open to examination by the Board or its authorized representative. Upon request of a producer, the purchaser or receiver of milk or cream, or both, shall permit the producer to examine the part of the record containing information concerning the samples of milk or cream representing the milk or cream delivered by the producer. A purchaser or receiver of milk or cream from the producer thereof shall, on written request, at least once each month mail or deliver to the producer[, at each time a list is made,] a written statement, unless the producer agrees to accept a verbal statement, of the percentage of milk [fat] components found to have been contained in the sample or samples representing the milk or cream delivered by the producer.
§ 144.14. Responsibility for violations.
[The] A certified tester at a laboratory or plant shall be responsible for a violation of the act or this chapter, including the keeping of the reports and records required by the act and this chapter. Additionally, the purchaser or receiver, or both, of the milk or cream, or both, or the licensed manager of a milk-gathering station, manufactory or plant receiving or purchasing milk or cream from producers for sale or resale or for manufacture, where the payment or settlement for the milk or cream is based in whole or in part on the milk [fat] component content thereof, [is] shall be responsible for a violation of the act or this chapter by a person working under his direction or subject to his orders or the act or this chapter, including the keeping of the reports and records [of the milk fat tests] required by the act and this chapter.
[Pa.B. Doc. No. 11-682. Filed for public inspection April 22, 2011, 9:00 a.m.]
[41 Pa.B. 2130]
[Saturday, April 23, 2011]


No part of the information on this site may be reproduced for profit or sold for profit.This material has been drawn directly from the official Pennsylvania Bulletin full text database. Due to the limitations of HTML or differences in display capabilities of different browsers, this version may differ slightly from the official printed version.