[48 Pa.B. 6791]
[Saturday, October 27, 2018]
[Continued from previous Web Page]
ADMINISTRATIVE CONTROLS § 221.11. Registrant responsibilities.
(a) The registrant is responsible for directing the operation of X-ray systems under his administrative control and shall assure that the requirements of this article are met in the operation of the X-ray systems.
(b) An individual who operates an X-ray system shall be instructed adequately in the safe operating procedures and be competent in the safe use of the equipment. The instructions shall include items included in Appendix A (relating to determination of competence) and there shall be continuing education in radiation safety, biological effects of radiation, quality assurance and quality control.
(1) The operator or the individual who supervises the operation of a high-risk procedure shall have additional instruction, which may include certification or registration in the applicable specialty by a professional organization recognized by the Department. Continuing education for high-risk procedures shall occur, at a minimum, every 2 years.
(2) Continuing education for all other (low-risk) procedures shall occur, at a minimum, every 4 years.
(c) Protocol information, which specifies the techniques for examinations performed with the system, shall be provided in the vicinity of each diagnostic X-ray system's control panel. The protocol shall include information pertinent to the particular examination, such as:
(1) The patient's body part and anatomical size, or body part thickness, or age (for pediatrics), versus technique factors to be utilized.
(2) The type and size of the image receptor or film-screen combination.
(3) The type of grid, if any.
(4) The type and location of placement of patient shielding, for example, gonad, and the like.
(5) For mammography, indication of kVp/target/filter combination.
(6) Source to image receptor distance to be used, except for dental intraoral radiography.
* * * * * (l) The registrant shall have a quality assurance program. This quality assurance program shall be documented and be in accordance with guidelines established by the Department or by another appropriate organization recognized by the Department. At a minimum, the quality assurance program shall address repeat rate, DRLs, image recording, processing and viewing, image quality and artifacts, and maintenance and modifications to the quality assurance program. For CT, each study shall be checked. If an artifact is present, the registrant shall take corrective action as appropriate. Records shall be maintained by the registrant for inspection by the Department for 5 years. The Department's guidelines and a list of recognized organizations will be maintained and made available on the Department's website and on request.
(m) Neither the X-ray tube housing nor the collimating device may be handheld during the exposure unless specifically designed to be handheld.
(n) Functional damage to a patient organ or a physiological system that results from a prescribed causative procedure shall be reported to the Department as outlined in § 219.229 (relating to diagnostic or interventional procedure medical reports).
(o) The registrant shall maintain records documenting the QMP's qualifications and compliance with continuing education requirements.
§ 221.16. Training, competency and continuing education.
(a) Training and competency. The registrant shall ensure that:
(1) An individual who operates X-ray equipment during diagnostic or interventional procedures or supervises the operation of X-ray equipment during a procedure is trained and competent in all of the following subject areas, as applicable to the procedures performed and the specific equipment utilized:
(i) Basic properties of radiation.
(ii) Units of measurement.
(iii) Sources of radiation exposure.
(iv) Methods of radiation protection for patients and others.
(v) Biological effects of radiation exposure.
(vi) Facility-specific and modality-specific X-ray equipment.
(vii) Facility-specific and modality-specific image recording and processing.
(viii) Patient exposure and positioning.
(ix) Facility-specific and modality-specific procedures.
(x) Facility-specific and modality-specific quality assurance.
(xi) Facility-specific and modality-specific dose reduction, monitoring and recording procedures.
(xii) Units of measurement and dose, such as dose-area product values, CT dose index and air kerma.
(xiii) Factors affecting fluoroscopic outputs.
(xiv) High-level control options.
(xv) Dose management including dose reduction techniques, monitoring and recording.
(xvi) Principles and operation of the specific fluoroscopic X-ray system to be used.
(xvii) Fluoroscopic and fluorographic outputs of each mode of operation on the system to be used clinically.
(xviii) Applicable State and Federal regulations.
(2) An individual who operates X-ray equipment during potentially high-risk diagnostic or interventional procedures or supervises the operation of X-ray equipment during these procedures is registered or credentialed and privileged in the applicable specialty by a professional organization recognized by the Department.
(3) Documentation demonstrating compliance with this section is maintained for inspection by the Department.
(b) Continuing education.
(1) The registrant shall ensure that individuals who operate X-ray equipment during diagnostic or interventional procedures or supervise the operation of X-ray equipment during a procedure complete continuing education in biological effects of radiation, quality assurance and quality control, and radiation safety, including concepts for minimizing patient and occupational dose and emerging technologies.
(i) An individual who performs low-risk procedures shall complete continuing education every 4 years.
(ii) An individual who performs high-risk procedures shall complete continuing education every 2 years. In addition to the topics in this paragraph, the continuing education must include facility and X-ray unit-specific methods to manage patient dose.
(2) Documentation of continuing education must be maintained for inspection by the Department for 5 years.
DIAGNOSTIC INSTALLATIONS GENERAL REQUIREMENTS § 221.21. Diagnostic equipment requirements.
(a) Diagnostic systems incorporating one or more certified components shall comply with 21 CFR 1020.30—1020.33.
(b) Equipment registered after _____ , (Editor's Note: The blank refers to the effective date of adoption of this final rulemaking.) must comply with 21 CFR 1010.2 (relating to certification).
§ 221.25. Beam quality.
(a) Diagnostic X-ray systems shall have filtration that satisfies the requirements of Table I. The requirements of this section shall be considered to have been met if it can be demonstrated that the half value layer of the primary beam is not less than that shown in Table II.
TABLE I
Filtration Required vs. Operating Voltage
Operating Voltage (kVp) Total Filtration (inherent plus added)
(millimeters aluminum equivalent)Below 50 0.5 millimeters 50—70 1.5 millimeters Above 70 2.5 millimeters
TABLE II
X-Ray Tube Voltage (kilovolt peak)
Design Operating Range Measured Operating Potential Minimum HVL (mm of Aluminum) Specified Dental
Systems1Other X-Ray
Systems2Other X-Ray
Systems3Below 51 30 1.5 0.3 0.3 40 1.5 0.4 0.4 50 1.5 0.5 0.5 51 to 70 51 1.5 1.2 1.3 60 1.5 1.3 1.5 70 1.5 1.5 1.8 Above 70 71 2.1 2.1 2.5 80 2.3 2.3 2.9 90 2.5 2.5 3.2 100 2.7 2.7 3.6 110 3.0 3.0 3.9 120 3.2 3.2 4.3 130 3.5 3.5 4.7 140 3.8 3.8 5.0 150 4.1 4.1 5.4 1 Dental X-ray systems designed for use with intraoral image receptors and manufactured after December 1, 1980.
2 Dental X-ray systems designed for use with intraoral image receptors and manufactured before or on December 1, 1980, and all other X-ray systems subject to this section and manufactured before June 10, 2006.
3 All X-ray systems, except dental X-ray systems designed for use with intraoral image receptors, subject to this section and manufactured on or after June 10, 2006.
Note: Half-value layers for kilovoltages not listed in Table II may be determined by interpolation or extrapolation.
(b) Beryllium window tubes shall have a minimum of 0.5 millimeter aluminum equivalent filtration permanently installed in the useful beam.
* * * * * § 221.35a. Fluoroscopic X-ray systems.
(a) General requirements. Fluoroscopic X-ray systems shall use an image intensifier and, in addition to the requirements of §§ 221.1—221.34a, shall meet the requirements of §§ 221.36a—221.38a (relating to limitation of useful beam of fluoroscopic equipment; activation of fluoroscopic tube; and entrance exposure rate).
(b) Operator qualifications. In addition to the applicable sections of these regulations, the operation of a fluoroscopic X-ray system for clinical purposes is limited to:
(1) A licensed practitioner working within his scope of practice.
(2) A Department-recognized radiologist assistant working within his scope of practice and under the direct supervision of a licensed practitioner working within his scope of practice.
(3) An individual who passed the American Registry of Radiologic Technologists exam or equivalent, holds a valid certification and is under the personal supervision of a licensed practitioner working within his scope of practice.
(4) A medical resident, radiologist assistant or radiologic technology student in training who is under the personal supervision of a licensed practitioner working within his scope of practice.
(c) QMP evaluations. Fluoroscopic equipment shall be evaluated by or under the direction of a QMP within 30 days after installation and after any maintenance of the system that may affect the exposure rate. Thereafter, evaluations shall be made at intervals not to exceed 14 months from the date of the prior evaluation by or under the direction of a QMP. At a minimum, evaluations shall include all of the following:
(1) A measurement of entrance exposure rates over a representative range of attenuating materials in all modes clinically used, including fluoroscopy, high-level control, acquisition and CINE, when available. Measurements shall be performed with a dosimetry system calibrated within 2 years preceding the measurements. Records of these output measurements shall be maintained for 5 years for inspection by the Department. Measurements shall be made as follows:
(i) For systems without automatic exposure control, by utilizing an mA and kVp typical of the clinical use of the fluoroscopic system.
(ii) For systems with automatic exposure control, by utilizing sufficient attenuating material in the useful beam to produce an mA and kVp typical of the clinical use of the fluoroscopic system.
(2) A measurement and verification of compliance with maximum air kerma rate for fluoroscopy and high-level control, if available.
(3) An evaluation of high-contrast resolution and low-contrast resolution in both fluoroscopic and spot-film or digital acquisition modes.
(4) An evaluation of the operation of the 5-minute timer, warning lights, interlocks and collision sensors.
(5) An evaluation of the beam quality.
(6) An evaluation of the collimation in the fluoroscopy and spot-film or digital acquisition modes.
(7) An evaluation of the availability and accuracy of technique indicators and integrated radiation dose displays.
(8) An evaluation of any changes that may impact patient and personnel exposure.
(d) Additional requirements for facilities performing FGI.
(1) The registrant utilizing FGI studies shall establish and implement written procedures, or procedures documented in an electronic reporting system, that include all of the following:
(i) Identification of individuals who are authorized to use fluoroscopic systems for interventional purposes.
(ii) A method to be used to monitor patient radiation dose during FGI.
(iii) Dose notification levels, as appropriate, at which the physician is notified for actions that may be taken for patient safety.
(iv) SRDL values referencing or consistent with nationally-recognized standards.
(v) Actions to be taken for cases when an SRDL is exceeded, which may include patient follow-up.
(vi) A review of the established procedures at an interval not to exceed 12 months.
(2) Records of policies and procedures shall be maintained for inspection by the Department. If the registrant revises a policy or procedure, documentation shall be maintained that includes the justification for the revision.
(3) A record of radiation output information shall be maintained so the radiation dose to the skin may be estimated in accordance with established protocols. The record must include all of the following:
(i) Patient identification.
(ii) Type and date of examination.
(iii) Identification of the fluoroscopic system used.
(iv) Peak skin dose, cumulative air kerma or dose area product used if the information is available on the fluoroscopic system.
(4) If the peak skin dose, cumulative air kerma or dose area product is not displayed on the fluoroscopic system, records must include other information necessary to estimate the radiation dose to the skin in accordance with established protocol or one or more of the following:
(i) Fluoroscopic mode, such as high-level or pulsed mode of operation.
(ii) Cumulative fluoroscopic exposure time.
(iii) Number of films or recorded exposures.
(5) The registrant shall maintain records for 5 years for inspection by the Department.
§ 221.50. Facilities using CR or DR.
(a) When exposure indicators are available, the facility shall establish, document and post an acceptable range for the exposure values for examinations routinely performed at the facility. The indicated exposure values for each image shall be compared to the established range. Consistent deviations from established ranges shall be investigated, corrective actions taken as necessary and results documented.
(b) Facilities shall establish and follow an image QC program in accordance with the recommendations of a QMP, the system manufacturer or a nationally-recognized organization.
(c) Facilities other than dental, podiatric and veterinary shall complete phantom image evaluation using a phantom approved by a QMP, system manufacturer or the Department. The evaluation shall be completed on a quarterly basis and include, at a minimum, all of the following:
(1) Artifacts.
(2) Spatial resolution.
(3) Contrast/noise.
(4) Workstation monitors.
(5) Exposure indicator constancy.
(d) In addition to subsections (a)—(c), CR facilities shall erase all CR cassettes, at a minimum, on a weekly basis.
(e) Dental and podiatric facilities shall maintain and operate photostimulable storage phosphor and DDR systems in accordance with manufacturer specifications.
(f) The facility shall maintain records for 5 years for inspection by the Department.
OTHER SYSTEMS § 221.61. Radiation therapy simulation systems.
(a) Fluoroscopic systems used solely for radiation therapy simulations shall only comply with §§ 221.35a(a) and (b), 221.37a, 221.40a and 221.41a. The requirements in § 221.41a (relating to fluoroscopic timer) may also be satisfied if a means is provided to indicate the cumulative time that an individual patient has been exposed to X-rays. In this case, procedures shall require that the timer be reset between examinations.
(b) CT units used solely for therapy simulations shall comply with §§ 221.202(h)(1), (7) and (8) and 221.203 (relating to equipment requirements; and facility design requirements).
§ 221.63. Therapy imaging guidance systems.
(a) The QMP shall develop QC procedures and tolerances for therapy imaging guidance systems following nationally-recognized standards or those recommended by the manufacturer.
(b) If a system is a CBCT, it must conform to the requirements of § 221.64 (relating to CBCT).
§ 221.64. CBCT.
(a) The following radiation measurements shall be evaluated annually and as soon as practical after a component repair or change which, in the opinion of the QMP or QE, may affect the performance of the CBCT unit:
(1) Beam alignment. The X-ray field in the plane of the image receptor may not exceed beyond the edge of the image receptor by more than 2% of the SID, when the axis of the X-ray beam is perpendicular to the plane of the image receptor. In addition, the center of the X-ray field must be aligned with the center of the image receptor to within 2% of the SID.
(2) A performance evaluation shall be performed by or under the direct supervision of a QMP or QE. The evaluation shall follow nationally-recognized standards and tolerances or those recommended by the manufacturer. The evaluation shall be performed within 30 days of initial installation, at intervals not to exceed 14 months, and within 30 days after any change or replacement of components which could cause a change in the radiation output or image quality.
(3) The registrant shall document and implement QC guidelines in accordance with nationally-recognized guidelines.
(4) The registrant shall document and implement a policy addressing deviations from established protocols.
(5) In addition to the requirements of § 221.16 (relating to training, competency and continuing education), the CBCT X-ray system shall only be operated by an individual who has been specifically trained in its operation.
(6) The facility shall maintain documentation of the established standards and tolerances and testing results for 5 years for inspection by the Department.
(b) The CBCT operator shall have instructions on all of the following:
(1) Performing routine QC, including the use of the CBCT phantom.
(2) A schedule of routine QC appropriate for the system.
(3) Allowable variations set by the QMP, if required, for the indicated parameters.
(4) The results of at least the most recent routine QC completed on the system.
(c) CBCT systems are exempt from § 221.202(a) (relating to equipment requirements).
§ 221.65. X-ray attenuation systems.
CT systems solely used to calculate attenuation coefficients or for image registration in nuclear medicine studies must meet the requirements in §§ 221.202—221.205 unless otherwise exempted as follows:
(1) CT systems identified in this section are exempt from §§ 221.202(a) and 221.204(a)(4)(xi) (relating to equipment requirements; and performance evaluations, routine QC and surveys).
(2) Instead of § 221.204(a) (relating to performance evaluations, routine QC and surveys), the registrant shall complete a performance evaluation on the CT system following the recommendations of a QMP, the system manufacturer or a nationally-recognized organization at intervals not to exceed 14 months.
(3) Instead of § 221.204(b), checks shall be established and documented by the registrant following nationally-recognized guidelines or those recommended by the manufacturer.
THERAPEUTIC X-RAY SYSTEMS WITH ENERGIES LESS THAN 1 MEV § 221.71. Equipment requirements.
* * * * * (m) Unless it is possible to bring the X-ray output to the prescribed exposure parameters within 5 seconds, the entire useful beam shall be automatically attenuated by a shutter having a lead equivalency not less than that of the tube housing assembly.
(1) After the unit is at operating parameters, the shutter shall be controlled electrically by the operator from the control panel.
(2) An indication of shutter position must appear at the control panel.
(n) Electronic brachytherapy devices are exempt from the requirements in subsections (k)—(m).
COMPUTED TOMOGRAPHY X-RAY SYSTEMS § 221.201. Definitions.
In addition to the definitions in §§ 215.2 and 221.2 (relating to definitions), the following words and terms, when used in this section and §§ 221.202—221.205, have the following meanings, unless the context clearly indicates otherwise:
Alert value—A dose index value (for example, CTDIvol (mGy) or of DLP (mGy-cm)) that is set by the registrant or licensee, or both, to trigger an alert to the operator prior to scanning within an ongoing examination. The alert value represents a value well above the registrant's or licensee's established range for the examination that warrants more stringent review and consideration before proceeding.
CS—Contrast scale—The change in the linear attenuation coefficient per CT number relative to water; that is:
CS = (Ux - Uw)/((CT)x - (CT)w)
Where:
Ux = Linear attenuation coefficient of the material of interest
Uw = Linear attenuation coefficient of water
(CT)x = CT number of the material of interest
(CT)w = CT number of water
CT—Computed tomography—The production of a tomogram by the acquisition and computer processing of X-ray transmission data.
CT conditions of operation—The selectable parameters governing the operation of a CT X-ray system including, but not limited to, nominal tomographic section thickness, filtration and the technique factors as defined in this chapter.
CT dosimetry phantom—The phantom used for determination of the dose delivered by a CT X-ray system.
CT number—The number used to represent the X-ray attenuation associated with each elemental area of the CT image:
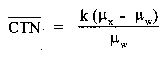
where:
k = A constant, a normal value of 1,000 when the Hounsfield scale of CTN is used.
µx = Linear attenuation coefficient of the material of interest.
µw = Linear attenuation coefficient of water.
CTDI—Computed tomography dose index—
(i) The integral of the dose profile along a line perpendicular to the tomographic plane divided by the product of the nominal tomographic section thickness and the number of tomograms produced in a single scan.
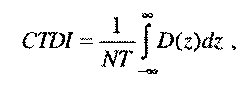
where:
z = Position along a line perpendicular to the tomographic plane.
D(z) = Dose at position z.
T = Nominal tomographic section thickness (cm).
N = Number of tomograms produced in a single scan.
(ii) This definition assumes that the dose profile is centered around z = 0 and that, for a multiple tomogram system, the scan increment between adjacent scans is NT.
CTDI100—An accumulated multiple scan dose at the center of a 100-mm scan that requires integration of the radiation dose profile from a single axial scan over specific integration limits. In the case of CTDI100, the integration limits are +50 mm, which corresponds to the 100-mm length of the commercially available ''pencil'' ionization chamber. CTDI100 is acquired using a 100-mm long, 3-cc active volume CT ''pencil'' ionization chamber, one of the two standard CTDI acrylic phantoms (16 and 32 cm diameter) and a stationary patient table.
CTDIvol—Volume Computed Tomography Dose Index—A radiation dose parameter derived from the CTDIw (weighted or average CTDI given across the field of view), that is:
CTDIvol = (N)(T)(CTDIw)/I,
where:
N = number of simultaneous axial scans per X-ray source rotation,
T = thickness of one axial scan (mm), and
I = table increment per axial scan (mm).
Thus,
CTDIvol = (1 / pitch) × CTDIw
CTDIw—Weighted Computed Tomography Dose Index—The estimated average CTDI100 across the field of view. The equation is:
CTDIw = 1/3 CTDI100.center + 2/3 CTDI100.edge
Where 1/3 and 2/3 approximate the relative areas represented by the center and edge values derived using the 16 cm or 32 cm acrylic phantom. CTDIw uses CTDI100 and an f-factor for air (0.87 rad/R for exposure or 1.0 mGy/mGy for air kerma measurements).
Detector—A device that provides a signal or other indication suitable for measuring one or more quantities of incident radiation.
Dose profile—The dose as a function of position along a line.
Elemental area—The smallest area within a tomogram for which the X-ray attenuation properties of a body are depicted.
Gantry—The tube housing assemblies, beam-limiting devices, detectors, transformers, if applicable, and the supporting structures and frames which hold these components.
Lux—A unit illumination equivalent to 1 lumen per square centimeter or 0.0929 foot-candles.
Modulation transfer function—The modulus of the Fourier transform of the impulse response of the system.
Multiple tomogram system—A CT X-ray system which obtains X-ray transmission data simultaneously during a single scan to produce more than one tomogram.
Noise—The standard deviation of the fluctuations in the CT number expressed as a percentage of the attenuation coefficient of water. Its estimate (Sn) is calculated using the following expression:
Sn = 100 × CS × S/Uw
Where:
CS = Contrast scale
Uw = Linear attenuation coefficient of water.
S = Estimated standard deviation of the CT number of picture elements in a specified area of the CT image.
Nominal tomographic section thickness—The full-width at half-maximum of the sensitivity profile taken at the center of the cross-sectional volume over which X-ray transmission data are collected.
Notification value—A dose index value (for example, CTDIvol (mGy) or DLP (mGy-cm)) that is set by the registrant to trigger a notification to the operator prior to scanning when the dose index exceeds the established range for the examination.
Performance phantom—A phantom which has a capability of providing an indication of CS, noise, nominal tomographic section thickness, the resolution capability of the CT system for low and high contrast objects, and measuring the mean CT number for water or other reference materials.
* * * * * § 221.202. Equipment requirements.
(a) Accreditation. All diagnostic CT X-ray systems must be accredited by an accrediting organization recognized by the Department within 1 year from first patient use.
(b) Technical and safety information. The technical and safety information relating to the conditions of operation, dose information and imaging performance provided by the CT manufacturer shall be maintained by the facility and readily accessible to the operators.
(c) Termination of exposure. The operator shall be able to terminate the X-ray exposure at any time during a scan, or series of scans under X-ray system control, of greater than 0.5 second duration. Termination of the X-ray exposure shall necessitate resetting of the conditions of operation prior to initiation of another scan.
(d) Tomographic plane indication and alignment.
(1) For any single tomogram system, a means shall be provided to permit visual determination of the tomographic plane or a reference plane offset from the tomographic plane.
(2) For any multiple tomogram system, a means shall be provided to permit visual determination of the location of a reference plane. This reference plane may be offset from the location of the tomographic plane.
(e) Status indicators and control switches.
(1) The CT X-ray control and gantry shall provide visual indication whenever X-rays are produced and, if applicable, whether the shutter is open or closed.
(2) The emergency buttons or switches shall be clearly labeled as to their function.
(3) Each individual scan or series of scans shall require initiation by the operator.
(f) Indication of CT conditions of operation. The CT X-ray system shall be designed so that the CT conditions of operation to be used during a scan or a scan sequence are indicated prior to the initiation of a scan or a scan sequence. On equipment having all or some of these conditions of operation at fixed values, this requirement may be met by permanent markings. Indication of CT conditions of operation shall be visible from any position from which scan initiation is possible.
(g) Leakage radiation. The leakage radiation from the diagnostic source assembly measured at a distance of 1 meter in any direction from the source may not exceed 100 milliroentgens (25.8 µC/kg) in 1 hour when the X-ray tube is operated at its leakage technique factors. Compliance shall be determined by measurements averaged over an area of 100 square centimeters with no linear dimension greater than 20 centimeters.
(h) Additional requirements applicable to CT X-ray systems containing a gantry manufactured after September 3, 1985.
(1) The total error in the indicated location of the tomographic plane or reference plane by the light field or laser indicator may not exceed 5 millimeters.
(2) If the X-ray production period is less than 0.5 second, the indication of X-ray production shall be actuated for at least 0.5 second. Beam-on and shutter status indicators at or near the gantry shall be discernible from any point external to the patient opening where insertion of any part of the human body into the primary beam is possible.
(3) The CT X-ray system shall be normalized to water.
(4) The CT number for water for a region of interest, not exceeding 100 square millimeters, shall be 0 ± 7.0 CT number units. The facility's performance phantom shall be utilized, with the technique factors specified by the QMP, to confirm compliance. In instances when a CTN of 0 for water is inappropriate, as in 3D treatment planning, the QMP may establish and maintain an equivalent value.
(5) With the performance phantom, the mean CT number of water of one group of pixels may not differ from the mean CT number of water of a second group of pixels equal size within the same image by more than the manufacturer's published specifications, or those established by the QMP.
(6) The noise, utilizing the facility's performance phantom, may not exceed the manufacturer's published specifications.
(7) The total error between the indicated and actual slice thickness may not exceed 2.0 millimeters.
(8) A distance of at least 100 millimeters measured in a CT image shall agree with the actual distance to within ± 5%.
(9) Premature termination of the X-ray exposure by the operator shall necessitate resetting the CT conditions of operation prior to the initiation of another scan.
§ 221.204. Performance evaluations, routine QC and surveys.
(a) Performance evaluations.
(1) The performance evaluation of the CT X-ray system shall be performed by or under the direction of a QMP.
(2) Evaluation standards and tolerances shall be established by a QMP and maintained by the facility. These standards and tolerances must meet nationally-recognized standards and tolerances for the CT X-ray system.
(3) The performance evaluation of a CT X-ray system shall be performed after initial installation and before use on human patients. Thereafter, the evaluation shall be made at intervals not to exceed 14 months.
(4) The performance evaluation must include all of the following:
(i) Geometric factors and alignment, including alignment light accuracy and table incrementation accuracy.
(ii) Slice localization from scanned projection radiograph (localization image).
(iii) Slice thickness.
(iv) Image quality including high-contrast (spatial) resolution, low-contrast resolution, image uniformity, noise and artifact evaluation.
(v) CT number accuracy.
(vi) Image quality for acquisition workstation display devices (video and hard copy when applicable).
(vii) A review of the results of the routine QC required under subsection (b).
(viii) A safety evaluation of audible and visual signals and posting requirements.
(ix) A review of commonly used CT protocols along with the evaluation for appropriateness of dose and image quality, in comparison with the older protocols. The review should be by the QMP along with the radiologist and lead CT technologist.
(x) For dosimetry, a review of the protocols deemed appropriate by the QMP which could result in significant doses. This review must include acquisition and reconstruction parameters, and radiation dose. At a minimum, the QMP shall review the following clinical protocols, if performed, at intervals not to exceed 14 months:
(A) Pediatric head (1 year of age).
(B) Pediatric abdomen (5 years of age; 40—50 lbs. (about 20 kg)).
(C) Adult head.
(D) Adult abdomen (70 kg).
(E) Brain perfusion.
(xi) Review DRL, notification values and alert values for the procedures reviewed under subparagraph (x).
(xii) Review actions to be taken when a dose alert value is exceeded including patient follow-up.
(xiii) Review the process determining who has access and authority to make changes to the protocol management systems, including a policy or procedure to prevent inadvertent or unauthorized modifications to a CT protocol.
(5) A performance evaluation shall be made within 30 days after any change or replacement of components which, in the opinion of the QMP, could cause a change in the radiation output or image quality.
(6) Dose measurements of a CT unit shall be performed with a calibrated dosimetry system. The calibration of the system shall be traceable to a national standard. The dosimetry system must have been calibrated within the preceding 2 years.
(b) Routine QC.
(1) Written routine QC procedures shall be developed by a QMP. These procedures shall be available for review by the Department.
(2) The routine QC procedures must include, at a minimum, all of the following using the facility's performance phantom:
(i) Noise.
(ii) Mean CT number for water.
(iii) Artifact evaluation.
(3) The routine QC shall be performed at intervals not to exceed 1 week.
(4) The QMP need not be present during the routine QC.
(5) Routine QC shall include acquisition of images obtained with the performance phantom using the same processing mode and CT conditions of operation as are used to perform the measurements required by subsection (a).
(c) Radiation protection surveys.
(1) CT X-ray systems shall have a survey performed at the time of installation by or under the direction of a QMP. In addition, a survey shall be performed after a change in the facility or equipment which might cause a significant increase in radiation hazard.
(2) The registrant shall obtain a written report of the survey from the QMP, and a copy of the report shall be made available to the Department upon request.
(d) Records. Records of the performance evaluations and surveys shall be maintained for inspection by the Department for at least 5 years. Routine QC records shall be maintained for at least 1 year.
§ 221.205. Operating procedures.
(a) In addition to the training requirements in § 221.16 (relating to training, competency and continuing education), a CT X-ray system shall be operated only by an individual who has been specifically trained in its operation.
(b) All of the following information must be readily available to the CT operator:
(1) Instructions on the use of the CT phantoms and a process for reporting deviations in protocols including a schedule of routine QC appropriate for the system, allowable variations for the indicated parameters and the results of at least the most recent performance evaluation conducted on the system.
(2) Current protocol information available at the control panel which specifies for each routine examination the CT conditions of operation.
(c) If the radiation measurements and performance evaluation of the CT X-ray system indicates that a system operating parameter has exceeded a tolerance established by the QMP, the use of the CT X-ray system on patients shall be limited to those uses permitted by established written instructions of the QMP.
CHAPTER 223. VETERINARY MEDICINE
GENERAL PROVISIONS § 223.1. Purpose and scope.
This chapter establishes radiation safety requirements for persons utilizing radiation sources in veterinary medicine. Persons who use radiation sources for veterinary medicine or research on animals shall comply with this chapter. The requirements of this chapter are in addition to and not in substitution for other applicable requirements of this article.
RADIOACTIVE MATERIAL § 223.22. Sealed and unsealed sources.
A veterinarian who uses sealed or unsealed sources for therapeutic treatment of animals shall comply with 10 CFR Parts 30 and 31.11 (relating to rules of general applicability to domestic licensing of byproduct material; and general license for use of byproduct material for certain in vitro clinical or laboratory testing).
ADMINISTRATIVE CONTROLS § 223.31. Registrant responsibilities.
(a) The registrant is responsible for directing the operation of X-ray systems under the registrant's administrative control and shall assure that the requirements of this article are met for the operation of the X-ray systems.
(b) A person who operates an X-ray system shall be instructed adequately about safe X-ray operating procedures and be competent in the safe use of X-ray equipment. The instructions must include the subjects listed in Chapter 221, Appendix A (relating to determination of competence). The person shall receive continuing education at least every 4 years in radiation safety, biological effects of radiation, species-specific positioning techniques, QA and QC.
(c) Written safety procedures and rules shall be available at the facility and include restrictions of the operating technique required for the safe operation of the particular X-ray system. The operator shall be able to demonstrate familiarity with these procedures and rules.
(d) Only the staff, ancillary facility personnel or other persons required for the medical procedure or training may be within 2 meters of the device during the radiographic exposure. All of the following requirements apply to persons involved with the examination:
(1) An individual or extremity may not be positioned in the useful beam unless required to conduct the procedure.
(2) Individuals shall be positioned so that no part of the body will be struck by the useful beam unless protected by at least 0.5 millimeter lead equivalent material. The lead equivalent of the material is to be determined at 60 kV.
(3) Each person shall be protected from stray radiation by protective aprons or whole protective barriers of at least 0.25 millimeter lead equivalent or shall be positioned so that no person is in the direct line of the useful beam and the nearest portion of the body is at least 2 meters from both the tube head and the nearest edge of the image receptor.
(e) If an animal or image receptor requires auxiliary support during a radiation exposure, all of the following requirements apply:
(1) Mechanical holding devices or chemical restraint shall be used when the technique permits.
(2) An individual may not be used routinely to hold image receptors or subjects. Procedures and auxiliary equipment designed to minimize personnel exposure commensurate with the needed diagnostic information shall be used.
(3) An individual who holds the animal or image receptor shall be protected as required under subsection (d).
(f) The registrant shall have a QA program. The QA program must be documented and be in accordance with guidelines established by the Department or by another appropriate organization recognized by the Department. At a minimum, the QA program must address radiation safety to personnel and modifications to the QA program.
(g) Neither the X-ray tube housing nor the collimating device may be handheld during the exposure unless specifically designed and shielded to be handheld.
(h) CT systems used solely for nonhuman imaging are exempt from §§ 221.202—221.205.
CHAPTER 224. MEDICAL USE OF RADIOACTIVE MATERIAL
Subchapter A. GENERAL § 224.11. Effect of incorporation of 10 CFR Part 35.
To reconcile differences between this chapter and the incorporated sections of 10 CFR Part 35 (relating to medical use of byproduct material), the following words and phrases shall be substituted for the language in 10 CFR Part 35 as follows:
(1) A reference to ''NRC'' or ''Commission'' means Department.
(2) A reference to ''NRC or agreement state'' means Department, NRC or agreement state.
(3) A reference to ''byproduct material'' includes NARM.
(4) The definition of ''sealed source'' includes NARM.
(5) A reference to the Advisory Committee on the Medical Uses of Isotopes is synonymous with the Department's Radiation Protection Advisory Committee.
(6) Notifications, reports and correspondence referenced in the incorporated parts of 10 CFR shall be directed to the Department.
CHAPTER 225. RADIATION SAFETY REQUIREMENTS FOR INDUSTRIAL RADIOGRAPHIC OPERATIONS
Subchapter A. GENERAL PROVISIONS § 225.3a. Effect of incorporation of 10 CFR Part 34.
To reconcile differences between this chapter and the incorporated sections of 10 CFR Part 34 (relating to licenses for industrial radiography and radiation safety requirements for industrial radiographic operations), the following words and phrases shall be substituted for the language in 10 CFR Part 34 as follows:
* * * * * (5) Notifications, reports and correspondence referenced in the incorporated parts of 10 CFR (relating to energy) shall be directed to the Department.
§ 225.4a. Radiation safety program.
(a) A person who intends to use radiation-producing machines for industrial radiography shall have a program for training personnel, written operating procedures and emergency procedures, individual monitoring reports required under 10 CFR 20.2206(a)(2) (relating to reports of individual monitoring), an internal review system and an organizational structure for radiographic operations which includes specified delegations of authority and responsibility for operation of the program. This program shall be approved by the Department before beginning industrial radiographic operations.
(b) The registrant shall notify the Department of intended changes to the registrant's radiation safety program and obtain Departmental approval.
Subchapter B. RADIATION-PRODUCING MACHINES
GENERAL TECHNICAL REQUIREMENTS § 225.81. Permanent radiographic installations.
(a) Permanent radiographic installations having high radiation area entrance controls of the types described in 10 CFR 20.1601 and 20.1902 (relating to control of access to high radiation areas; and posting requirements) shall also meet all of the following requirements:
(1) Each entrance that is used for personnel access to the high radiation area in a permanent radiographic installation shall have both visible and audible warning signals to warn of the presence of radiation. The visible signal shall be activated by radiation whenever the X-ray source is energized. The audible signal shall be actuated when an attempt is made to enter the installation while the X-ray source is energized.
(2) The entrance control device or alarm system shall be tested for proper function prior to beginning operations on each day of use.
(3) The radiographic exposure system may not be used if an entrance control device or alarm system is not operating properly. If an entrance control device or alarm system is not functioning properly, it shall be removed from service and repaired or replaced immediately. If no replacement is available, the facility may continue to be used provided that the registrant implements the continuous surveillance under 10 CFR 34.51 and 34.53 (relating to surveillance; and posting), § 225.83 (relating to records required at field radiography sites) and uses an alarming ratemeter. Before the entrance control device or alarm system is returned to service, the radiation safety officer or an individual designated by the radiation safety officer shall validate the repair.
(b) Records of the tests performed under subsection (a) shall be maintained for inspection by the Department for 5 years.
CHAPTER 226. LICENSES AND RADIATION SAFETY REQUIREMENTS FOR WELL LOGGING
GENERAL § 226.5. Effect of incorporation of 10 CFR Part 39.
To reconcile differences between this chapter and the incorporated sections of 10 CFR Part 39 (relating to licenses and radiation safety requirements for well logging), the following words and phrases shall be substituted for the language in 10 CFR Part 39 as follows:
(1) A reference to ''NRC'' or ''Commission'' means Department.
(2) A reference to ''NRC or agreement state'' means Department, NRC or agreement state.
(3) The definition of ''sealed source'' includes NARM.
(4) The definition of ''licensed material'' includes NARM.
(5) Notifications, reports and correspondence referenced in the incorporated parts of 10 CFR (relating to energy) shall be directed to the Department.
CHAPTER 227. RADIATION SAFETY REQUIREMENTS FOR ANALYTICAL X-RAY EQUIPMENT, X-RAY GAUGING EQUIPMENT, ELECTRON MICROSCOPES AND X-RAY CALIBRATION SYSTEMS
ANALYTICAL X-RAY EQUIPMENT § 227.11a. Equipment requirements.
* * * * * (h) Equipment exclusively designed and exclusively used for vacuum spectroscopy where the tube housing and sample chamber is located behind all external surfaces of the unit shall be exempt from the requirements of this section, §§ 227.12a and 227.13a (relating to area requirements; and operating requirements), but shall meet the requirements of § 227.14 (relating to personnel requirements) and the following:
* * * * * (6) A sign bearing the radiation symbol and the words ''CAUTION—RADIATION,'' or words of similar intent shall be placed next to the opening of the sample chamber.
(i) Analytical X-ray equipment operating at less than or equal to 50 kV tube voltage and designed to be held by an operator during use are exempt from the requirements of this section and § 227.12a(b), but shall meet the requirements of subsection (f)(2) and §§ 227.13a(a) and 227.14(a).
CHAPTER 228. RADIATION SAFETY REQUIREMENTS FOR PARTICLE ACCELERATORS
ADMINISTRATIVE CONTROLS § 228.11a. Licensee responsibilities.
(a) A person may not possess, operate or permit the operation of an accelerator unless the accelerator and installation meet the applicable requirements of this article.
(b) Written safety procedures and rules shall be available at a facility, including restrictions of the operating technique required for the safe operation of the particular accelerator. The operator shall be able to demonstrate familiarity with the rules. The operator of an accelerator used for healing arts shall have additional instruction, including certification in the applicable specialty by a professional organization recognized by the Department.
(c) An individual may not be exposed to the useful beam except for healing arts purposes. An exposure shall be authorized by a licensed practitioner of the healing arts.
NOTIFICATION AND LICENSING PROCEDURES § 228.21a. Notification and license requirements.
(a) A person who intends to purchase, construct or acquire an accelerator shall notify the Department of this intent by filing an application for a specific license within 90 days after the initial order is issued to obtain any or all parts of the accelerator.
(1) The application shall be filed in duplicate on a form prescribed by the Department and shall be accompanied by the required fee as described in § 218.11(d) (relating to registration, renewal of registration and license fees).
(2) The application shall contain pertinent information to permit the Department to evaluate the accelerator facility for compliance with the act and this article.
(b) In addition to the notification requirement in subsection (a), a person who intends to install an accelerator shall notify the Department within 30 days after the initial construction or installation begins.
(c) The Department may, after the filing of an original application, and before the expiration of the license, require further information to enable the Department to determine whether the application will be granted or denied or whether a license will be modified or revoked.
(d) The application shall be signed by the applicant or licensee, or an individual authorized by the applicant or licensee.
(e) A license issued under this chapter may not be transferred, assigned or in any manner disposed of, either voluntarily or involuntarily, to any person except through submission of a written request by the licensee to the Department for approval.
GENERAL RADIATION SAFETY REQUIREMENTS § 228.35. Operating procedures.
* * * * * (c) Each safety and warning device, except interlocks, shall be checked at least every 3 months for proper functioning and shall be repaired as necessary. Interlocks shall be checked at least annually. Results of these checks and records of repairs shall be maintained for 5 years at the accelerator facility for inspection by the Department.
* * * * * (g) For accelerators used in the healing arts, operating procedures shall meet the following requirements:
* * * * * (h) An individual who operates an accelerator system shall be instructed adequately in the safe operating procedures and be competent in the safe use of the equipment. The instructions must include items included in Appendix A (relating to determination of competence) for medical accelerator operations, as well as basic radiation protection for nonmedical accelerator operations. There shall be continuing education in radiation safety, biological effects of radiation, quality assurance and quality control.
§ 228.36. Radiation monitoring requirements.
An independent radiation monitoring system shall be provided so that the individuals entering or present in a potential very high radiation area become aware of the existence of the hazard. Independent radiation monitors shall be tested for response daily and after each servicing or repair.
RADIATION SAFETY REQUIREMENTS FOR ACCELERATORS USED IN THE HEALING ARTS § 228.61. Leakage radiation to the patient area.
(a) Equipment must meet all of the following requirements:
(1) For operating conditions producing maximum leakage radiation, the dose due to leakage radiation, including X-rays, electrons and neutrons, at any point on a circle of 2 meters radius centered on and perpendicular to the central axis of the beam at the isocenter or normal treatment distance and outside the maximum useful beam size, may not exceed 0.1% of the maximum dose of the unattenuated useful beam measured at the point of intersection of the central axis of the beam and the plane surface. Measurements, excluding those for neutrons, shall be averaged over an area up to, but not exceeding, 100 square centimeters at the position specified. Measurements of the portion of the leakage radiation dose contributed by neutrons shall be averaged over an area up to, but not exceeding, 200 square centimeters.
(2) For each system, the licensee shall determine or obtain from the manufacturer the leakage radiation existing at the positions specified in paragraph (1) for the specified operating conditions. The licensee shall maintain records for 5 years on leakage radiation measurements for inspection by the Department.
(b) Equipment manufactured or installed prior to July 17, 2004, must meet all of the following requirements:
(1) For operating conditions producing maximum leakage radiation, the absorbed dose due to leakage radiation, including neutrons, at any point on a circle of 2 meters radius centered on and perpendicular to the central axis of the beam 1 meter from the virtual source, may not exceed 0.1% of the maximum absorbed dose of the unattenuated useful beam measured at the point of intersection of the central axis of the beam and the surface of the circular plane. Measurements shall be averaged over an area up to but not exceeding 100 square centimeters at the positions specified.
(2) For each system, the licensee shall have available the leakage radiation data existing at the positions specified in paragraph (1) for the specified operating conditions. The licensee shall maintain records on radiation leakage for 5 years for inspection by the Department.
§ 228.72. Selection of radiation type.
Equipment capable of X-ray therapy or electron therapy, or both, must meet all of the following additional requirements:
* * * * * § 228.73. Selection of stationary beam therapy or moving beam therapy.
Equipment capable of stationary beam therapy or moving beam therapy, or both, must meet all of the following additional requirements:
* * * * * § 228.75. Calibrations.
* * * * * (e) The calibration of the therapy beam shall include, but is not limited to, the following determinations:
(1) Verification that the equipment is operating in compliance with the design specifications concerning the light localizer, the side light and back-pointer alignment with the isocenter when applicable, variation in the axis of rotation for the table, gantry and beam limiting device (collimator) system.
(2) The absorbed dose rate at various depths (depth dose) and beam profile measured in water and the beam flatness and symmetry for the range of field sizes used, for each beam energy, and if applicable, for each flattening filter free mode.
* * * * *
CHAPTER 230. PACKAGING AND TRANSPORTATION OF RADIOACTIVE MATERIAL
Subchapter A. SCOPE AND DEFINITIONS § 230.4. Effect of incorporation of 10 CFR Part 71.
To reconcile differences between this chapter and the incorporated sections of 10 CFR Part 71 (relating to packaging and transportation of radioactive material), the following words and phrases shall be substituted for the language in 10 CFR Part 71 as follows:
(1) A reference to ''NRC'' or ''Commission'' means Department.
(2) A reference to ''NRC or agreement state'' means Department, NRC or agreement state.
(3) The definition of ''sealed source'' includes NARM.
(4) The definition of ''licensed material'' includes NARM.
(5) Notifications, reports and correspondence referenced in the incorporated parts of 10 CFR (relating to energy) shall be directed to the Department.
Subchapter B. GENERAL § 230.15. Packaging and transportation of unlicensed material.
Radioactive material not licensed by the Department or under the specific regulatory control of another state or Federal agency that meets the definition of radioactive material in 49 CFR 173.403 (relating to definitions) must be packaged and transported in compliance with the standards and requirements of 49 CFR 173.401—173.477 (relating to class 7 (radioactive) materials).
CHAPTER 232. LICENSES AND RADIATION SAFETY REQUIREMENTS FOR IRRADIATORS § 232.3. Effect of incorporation of 10 CFR Part 36.
To reconcile differences between this chapter and the incorporated sections of 10 CFR Part 36 (relating to licenses and radiation safety requirements for irradiators), the following words and phrases shall be substituted for the language in 10 CFR Part 36 as follows:
(1) A reference to ''NRC'' or ''Commission'' means Department.
(2) A reference to ''NRC or agreement state'' means Department, NRC or Agreement State.
(3) The definition of ''sealed source'' includes NARM.
(4) Notifications, reports and correspondence referenced in the incorporated parts of 10 CFR (relating to energy) shall be directed to the Department.
CHAPTER 240. RADON CERTIFICATION
Subchapter A. GENERAL PROVISIONS
GENERAL § 240.1. Description of regulatory structure.
* * * * * (e) Subchapter E (relating to enforcement and decertification) contains the enforcement provisions, including inspection, decertification and assessment of civil penalties. Other enforcement actions are available under sections 308 and 309 of the Radiation Protection Act (35 P.S. §§ 7110.308 and 7110.309) and section 14 of the act (63 P.S. § 2014).
(f) This section is for descriptive purposes only. This section does not limit the authority of the Department under the acts or this chapter.
§ 240.2. Scope.
(a) This chapter applies to a person except when the person is:
(1) Testing for or mitigating against radon contamination in a building that the person owns or in which the person resides.
(2) Using measures designed to prevent radon contamination in newly constructed buildings. This exemption does not apply to radon testing or installation of radon mitigating devices in these buildings following occupancy.
(3) Performing testing or mitigation in the course of the person's normal duties as an employee or contractor of the Department or the Federal government.
(4) Performing scientific research if the person discloses the information obtained to the Department under § 240.303 (relating to reporting of information) and the person informs the owner or occupant of the affected building of all of the following:
(i) That the person is not certified by the Department to test for or mitigate against radon contamination.
(ii) That the test results are not valid.
(iii) That the mitigation methods are for experimental purposes and may be unsuccessful.
(5) Purveying secondary devices supplied by a certified laboratory, if radon concentrations determined by the laboratory are only reported directly to the owner or resident of the building tested.
(i) Test results may also be reported to the certified mitigator who installed a mitigation system at the property.
(ii) Purveying does not include the activities of either placing or retrieving activated charcoal, liquid scintillation, or alpha track radon testing devices.
(6) Employed by a local government or a school and performing testing for that local government or school if all of the following criteria are met:
(i) The practice is limited to the employee's official duties and no fee is charged for the testing except for the employee's salary.
(ii) Radon testing is limited to the buildings owned or occupied by the local government or school.
(iii) The radon testing is performed in accordance with the device manufacturer's instructions.
(b) This chapter is in addition to, and not in substitution for, other applicable provisions of this article.
§ 240.3. Definitions.
The following words and terms, when used in this chapter, have the following meanings, unless the context clearly indicates otherwise:
AC—Activated charcoal—A device used to measure radon by exposing activated charcoal to air in the area to be tested and analyzed by gamma ray spectroscopy.
AT—Alpha track—A device used to measure radon by recording alpha particle tracks on a plastic chip.
Act—The Radon Certification Act (63 P.S. §§ 2001—2014).
Active radon mitigation system—A radon mitigation system with an electric vent fan.
Acts—The Radon Certification Act and the Radiation Protection Act (35 P.S. §§ 7110.101—7110.703).
Alteration—A change to the original mitigation system design, including fan size, number or placement of suction points, or pipe diameter.
CRM—Continuous radon monitor—An active device used to measure radon with solid state silicon surface barrier detectors, scintillation cells or ion chambers, usually on an hourly basis.
CWLM—Continuous working level monitor—An active device used to measure radon decay products, usually on an hourly basis.
Calibration—The process of determining the response of an instrument (or measurement system) to a series of known values over the range of the instrument (or measurement system).
Certification year—Each 12-month period beginning with the most recent certification date of the certified individual.
Certified individual—An individual with a Department certification to perform radon testing, mitigation or laboratory analysis in this Commonwealth.
Client—A receiver of services that are regulated under the Act or this chapter.
Control limit—A QC value set at ±3 sigma.
Diagnostic test—A test performed to determine specific radon entry points and sources, the result of which is not reported to the Department or in writing to the client.
Duplicate measurements—Two measurements made concurrently, for the same time period and in the same location, approximately 4 inches from one another.
Electret ion chamber—A radon measurement device that consists of a small plastic container with an electrostatically charged disk inside to serve as a detector.
Electret reader—A radon measurement device that consists of a voltmeter used to measure the voltage on the electrostatically charged disk of an electret ion chamber testing device at the beginning and end of a test period.
Electret voltage drift—A QC process which evaluates the voltage drift of each new batch of electrets received from the manufacturer of the electrets.
Field blank—A QC measurement made by analyzing unexposed (closed) detectors that have been maintained in a low-radon environment to assess radon exposure to the detector from a source other than the concentration in the environment to be measured.
Firm—A Department-certified entity that has at least one certified individual in responsible charge of the entity's testing, mitigation or laboratory radon activities. A business, such as a corporation or limited liability company, may contain more than one firm.
Firm employee—A Department-listed radon testing, mitigation or laboratory employee under the responsible charge of a certified individual.
Firm owner—A person or business entity which owns and is responsible for the radon firm.
LS—Liquid scintillation—A device used to measure radon by exposing a small amount of activated charcoal contained within a small vial and placed in the area to be sampled and analyzed in a liquid scintillation counter.
Laboratory—A Department-certified individual or firm.
Laboratory analysis—The act of analyzing a radon test device and calculating a radon concentration in air or water.
Lowest livable level—The lowest level of a building that may be used as a living space without requiring any major structural changes.
MV—Measured value—The radon concentration reported by the analyst, in units of picocuries per liter or WLs.
Measurement—A radon or radon decay product test result used for the performance of quality assurance, including a spike, blank, duplicate, intercomparison or cross check.
Mitigate—To repair or alter a building or building design for the purpose in whole or in part of reducing the concentration of radon in the indoor atmosphere.
Mitigator—A Department-certified individual or a Department-listed mitigation employee of a Department-certified mitigation firm.
Multifamily building—A building with more than three attached dwellings.
Nonreported test—A test conducted for reasons other than reporting valid, written results to the client, such as a diagnostic test.
pCi/L—Picocurie per liter—2.22 disintegrations per minute of radioactive material per liter of air.
Passive radon mitigation system—A radon mitigation system without an electric vent fan.
Person—An individual, corporation, partnership, business entity, association, trust, estate, public or private institution, group, agency or political subdivision of this Commonwealth, another state or political subdivision or agency thereof, and a legal successor, representative, agency or agency of the entities in this definition.
Primary device—Continuous monitors or electret ion chambers, or both, read or analyzed, or both, by a primary tester.
Primary tester—A tester who reads or analyzes, or both, a primary device that the tester places or retrieves, or both.
QA—Quality assurance—The activities required to provide the evidences needed to establish confidence that radon test data are of the required precision and accuracy.
QC—Quality control—The process through which a person measures performance, compares performance with standards and acts on any differences.
RPD—Relative percent difference—The absolute value of the difference between two measurements divided by their average, multiplied by 100. The equation is:
RPD={(¦MV1-MV2¦)/(MV1+MV2)/2} × 100.
RPE—Relative percent error—The measured value (pCi/L) minus the RV (pCi/L), divided by the RV, multiplied by 100. The equation is:
RPE = {(MV—RV)/RV} × 100.
RV—Reference value—The known radon concentration value, in units of picocuries per liter or WL, to which a test device is exposed.
Radon—The radioactive noble gas radon-222 and the short-lived radionuclides which are products of radon-222 decay, including polonium-218, lead-214, bismuth-214 and polonium-214.
Secondary device—A radon test device that is analyzed by a Department-certified laboratory.
Secondary tester—A tester who places or retrieves, or both, a radon test device that is analyzed by a Department-certified laboratory.
Sigma level—A sample standard deviation around a mean, which is a measure of the scatter of data around a mean. The term is often described as 1, 2 or 3 sigma, corresponding to one, two or three standard deviations around the mean.
Spiked measurement or spike—A quality control measurement conducted in an approved chamber to evaluate accuracy by exposing the detector or device to a known concentration and submitted for analysis.
Test—The act of measuring for the presence of radon in a building's air or water supply.
Tester—A Department-certified individual or a Department-listed testing employee of a Department-certified testing firm.
WL—Working level—Any combination of short-lived radon progeny (for radon-222: polonium-218, lead-214, bismuth-214 and polonium-214; and for radon-220: polonium-216, lead-212, bismuth-212 and polonium-212) in 1 liter of air that will result in the ultimate emission of 1.3 × 105 MeV of alpha particle energy.
WLM—Working level month—The cumulative exposure from breathing in an atmosphere at a concentration of 1 WL for a working month of 170 hours.
WLM/yr—Working level month per year—The cumulative exposure incurred over 1 year (2,040 hours) from breathing in an atmosphere at a concentration of 1 WL for a working month of 170 hours.
Warning level—A QC value set at ±2 sigma.
Subchapter B. CERTIFICATION
CERTIFICATION FOR RADON TESTING § 240.101. Requirements for radon testing certification.
(a) A person may not test for radon or represent or advertise that he may so test in a building in this Commonwealth unless the person has first applied for and obtained certification from the Department to test or is a firm employee of a certified testing firm.
(b) For a firm to perform radon testing it shall employ at least one individual certified to test who is in responsible charge of the firm's testing activities, and the firm shall submit an application for certification and receive certification from the Department.
(c) A certified primary tester does not also have to be certified in radon laboratory analysis to read or analyze continuous monitors or electret ion chambers that he places and retrieves.
(d) A person using secondary radon testing devices, such as AC, from a certified radon laboratory does not also have to be certified in radon laboratory analysis.
§ 240.102. Prerequisites for radon testing certification.
(a) Individual certification for radon testing. An individual will not be certified to test unless the individual has:
(1) Completed a Department-approved course on radon.
(2) Passed a Department-approved written exam on radon testing within 2 years before the postmark date of the individual's application submittal. The applicant shall forward a copy of exam results to the Department.
(3) Submitted a complete and accurate application to the Department, including applicable fees.
(b) Firm certification for radon testing. If the applicant for testing certification is a firm, it shall employ at least one individual who is certified to test and who is in responsible charge of the firm's testing activities.
(1) If the firm loses its certified individual, all of the following apply:
(i) The firm owner shall notify the Department in writing within 5 business days of losing that individual.
(ii) The firm's certification automatically lapses and is void until the Department approves in writing the firm owner's written and signed request for a certified individual to be in responsible charge of that firm's radon testing activities.
(2) If a testing firm employee is no longer under the responsible charge of the firm's certified individual, all of the following apply:
(i) The firm's certified individual shall notify the Department within 10 business days of this change.
(ii) The firm employee's Department listing becomes invalid.
(3) Each testing firm employee shall conduct activities in accordance with the signed testing firm employee application.
(4) Each testing firm employee applicant shall submit all of the following:
(i) A nonrefundable fee as set forth in Appendix A (relating to radon certification fee schedule).
(ii) A completed firm employee application as provided by the Department within 10 business days of performing radon testing activities.
(iii) For firm employees hired after January 24, 2019, a certification that the firm employee received initial training pursuant to subsection (b)(6).
(iv) A document signed by the certified individual that the firm employee completed continuing education as required by subsection (b)(7), if applicable.
(v) The applicant's current photograph, in a format specified by the Department, to be used on the identification card as required under § 240.142 (relating to testing and mitigation identification cards).
(5) The firm's certified individual shall receive written approval from the Department of a testing firm employee.
(6) For firm employees hired after January 24, 2019, the firm's certified individual shall ensure that each firm employee receives initial training before participating in radon testing activities. Initial training may be given by the firm's certified individual or through a department-approved training program. The firm's certified individual shall document that each firm employee has received initial training that includes, at a minimum, the following:
(i) General information regarding radon and the risks associated with radon exposure.
(ii) A tutorial on how to properly use the testing device(s) employed by the certified firm including:
(A) The strengths and weaknesses of the specific device(s) including any limitations of the device(s).
(B) Device handling precautions, if any.
(C) Short-term versus long-term testing.
(D) Device sampling times.
(E) When to invalidate a measurement.
(iii) Information regarding the appropriate radon testing protocol(s) including:
(A) Closed building conditions.
(B) Heating and air conditioning system considerations.
(C) Unusual weather conditions.
(D) Tampering precautions.
(E) Measurement documentation.
(F) Brief QA/QC overview.
(G) Real estate and non-real estate testing.
(H) Device placement locations within the building.
(7) The firm's certified individual shall ensure that each firm employee receives continuing education every two years. Continuing education may be given by the firm's certified individual or through a Department-approved training program. The firm's certified individual shall document that each firm employee has received continuing education. Continuing education records shall be retained for 5 years. Continuing education shall include, at a minimum, the requirements set forth in subsection (b)(6)(ii) and (iii).
(c) Additional requirements. If the applicant for testing certification is a firm, or an individual performing testing and not working for a certified radon testing firm, the applicant shall also have a QA program and a continuing education program as required under §§ 240.306 and 240.604 (relating to continuing education program; and QA requirements for testing using primary devices). In addition, the applicant shall be successfully enrolled in a Department-approved radon measurement proficiency program as required under § 240.307 (relating to radon measurement proficiency program).
§ 240.103. Radon testing application contents.
(a) An application for radon testing certification, by an individual or a firm, shall be submitted to the Department in writing on forms provided by the Department and must contain all of the following:
(1) Evidence that the applicant has the certification prerequisites in § 240.102 (relating to prerequisites for radon testing certification). The application must include the duties assigned to the certified individual in responsible charge of the testing activities.
(2) A nonrefundable fee as set forth in Appendix A (relating to radon certification fee schedule).
(3) The applicant's name, address, and telephone number. It must also indicate if the applicant is an individual, partnership, limited partnership, corporation or other entity. The application must include, when appropriate, the name and address of every officer, general and limited partner, director, principal shareholder, parent corporation and certified person within the applicant's organization.
(4) Compliance information, including descriptions of notices of violation, administrative orders, civil penalty assessments and actions for violations of the act, this chapter or a term or condition of a certification.
(5) Copies of reporting forms, information distributed to potential clients and recent or proposed advertisements.
(6) The applicant's current photograph, in a format specified by the Department, to be used on the identification card as required under § 240.142 (relating to testing and mitigation identification cards).
(7) Other information the Department may require related to an applicant's qualifications or technical or administrative information related to radon testing.
(8) A verification by the applicant that the information contained in the application is correct to the best of the applicant's information and belief. This verification is subject to the penalties of 18 Pa.C.S. § 4904 (relating to unsworn falsification to authorities).
(9) If the applicant for testing certification is a firm, the application shall include a demonstration that the firm's certified individual will maintain adequate span of control over the firm's employees. This demonstration shall include, at a minimum, the following:
(i) Information regarding the initial training and continuing education given to firm employees that is required by § 240.102(b)(6) and (b)(7) (relating to prerequisites for radon testing certification).
(ii) The firm's protocol for ensuring that firm employees are adequately supervised by the firm's certified individual.
(b) Within 10 business days of a change to the information submitted in the certified individual application or firm certification application, the certified individual shall submit to the Department a written and signed notification listing each change. The change will not take effect until the Department provides written approval of the change.
§ 240.104. Application filing deadline.
(a) A person who expects to conduct radon testing shall file a complete application for certification a minimum of 30 days prior to the anticipated starting date of testing activity.
(b) A testing individual certification renewal application postmarked after the previous testing individual certification expiration date will be charged a late application fee as set forth in Appendix A (relating to radon certification fee schedule).
CERTIFICATION FOR RADON MITIGATION § 240.111. Requirements for radon mitigation certification.
(a) A person may not mitigate radon contamination in a building or represent or advertise that he may so mitigate in a building in this Commonwealth unless the person has first applied for and obtained certification from the Department to mitigate or is a firm employee of a certified mitigation firm.
(b) For a firm to perform radon mitigation it shall employ at least one individual certified to mitigate who is in responsible charge of the firm's mitigation activities, and the firm shall submit an application for certification and receive certification from the Department prior to performing mitigation of radon contamination.
§ 240.112. Prerequisites for radon mitigation certification.
(a) Individual certification for radon mitigation. An individual will not be certified to mitigate unless the individual has:
(1) Completed a Department-approved course on radon mitigation.
(2) Passed a Department-approved written exam on radon mitigation within 2 years before the postmark date of the individual's application submittal. The applicant shall forward a copy of exam results to the Department.
(3) Had 1 year professional experience in radon mitigation system installation or 3 years experience in architecture, engineering, electrical contracting, plumbing, carpentry, masonry or related trades.
(4) Submitted a complete and accurate application to the Department including applicable fees.
(b) Firm certification for radon mitigation. If the applicant for mitigation certification is a firm, it shall employ at least one individual who is certified to mitigate and who is in responsible charge of the firm's mitigation activities.
(1) If the firm loses its certified mitigation individual, all of the following apply:
(i) The mitigation firm owner shall notify the Department in writing within 5 business days of losing that individual.
(ii) The firm's certification automatically lapses and is void until the Department approves in writing the mitigation firm owner's written and signed request for a certified individual to be in responsible charge of that firm's radon mitigation activities.
(2) If the mitigation firm employee is no longer under the responsible charge of the firm's certified individual, all of the following apply:
(i) The firm's certified individual shall notify the Department within 10 business days of this change.
(ii) The firm employee's Department listing becomes invalid.
(3) The mitigation firm employee shall conduct activities in accordance with the signed mitigation firm employee application.
(4) Each mitigation firm employee applicant shall submit all of the following:
(i) A completed firm employee application as provided by the Department within 10 business days of performing radon mitigation activities.
(ii) The applicant's current photograph, in a format specified by the Department, to be used on the identification card as required under § 240.142 (relating to testing and mitigation identification cards).
(iii) For firm employees hired after January 24, 2019, a certification that the firm employee received initial training pursuant to subsection (b)(6).
(iv) A document signed by the certified individual that the firm employee completed continuing education as required by subsection (b)(7), if applicable.
(5) The firm's certified individual shall receive written approval from the Department of a mitigation firm employee.
(6) For firm employees hired after January 24, 2019, the firm's certified individual shall ensure that each firm employee receives initial training before participating in radon mitigation activities. Initial training may be given by the firm's certified individual or through a Department-approved training program. The firm's certified individual shall document that each firm employee has received initial training that includes, at a minimum, the following:
(i) Information regarding radon and the risks associated with radon exposure.
(ii) Information regarding radon mitigation health and safety topics such as fall protection, mold hazards, and ventilation.
(iii) Information regarding radon mitigation protocols and standards.
(iv) Information regarding electrical wiring and electrical issues as they relate to radon mitigation installations.
(7) The firm's certified individual shall ensure that each firm employee receives continuing education every two years. Continuing education may be given by the firm's certified individual or through a Department-approved training program. The firm's certified individual shall document that each firm employee has received continuing education. Continuing education records shall be retained for 5 years. Continuing education shall include at least the requirements set forth in subsection (b)(6)(ii)-(iv).
(c) Additional requirements. If the applicant for mitigation certification is a firm, or an individual performing mitigation and not working for a certified mitigation firm, he shall also have a health and safety program, and a continuing education program, as required in §§ 240.305 and 240.306 (relating to health and safety program; and continuing education program).
§ 240.113. Radon mitigation application contents.
(a) An application for radon mitigation certification, by an individual or a firm, shall be submitted to the Department in writing on forms provided by the Department and must contain all of the following:
(1) Evidence that the applicant has the certification prerequisites contained in § 240.112 (relating to prerequisites for radon mitigation certification). The application must include the duties assigned to the certified individual in responsible charge of the mitigation activities.
(2) A nonrefundable fee as set forth in Appendix A (relating to radon certification fee schedule).
(3) The applicant's name, address, and telephone number. It must also indicate if the applicant is an individual, partnership, limited partnership, corporation or other entity. The application must include, when appropriate, the name and address of every officer, general and limited partner, director, principal shareholder, parent corporation and certified person within the applicant's organization.
(4) Compliance information, including descriptions of notices of violation, administrative orders, civil penalty assessments and actions for violations of the act, this chapter or a term or condition of a certification.
(5) Copies of reporting forms, information distributed to potential clients and recent or proposed advertisements.
(6) The applicant's current photograph, in a format specified by the Department, to be used on the identification card as required under § 240.142 (relating to testing and mitigation identification cards).
(7) Other information the Department may require related to an applicant's qualifications or technical or administrative information related to radon mitigation.
(8) A verification by the applicant that the information contained in the application is correct to the best of the applicant's information and belief. This verification is subject to the penalties of 18 Pa.C.S. § 4904 (relating to unsworn falsification to authorities).
(9) If the applicant for mitigation certification is a firm, the application shall include a demonstration that the firm's certified individual will maintain adequate span of control over the firm's employees. This demonstration shall at least include:
(i) Information regarding the initial training and continuing education given to firm employees that is required by § 240.112(b)(6) and (b)(7).
(ii) The firm's protocol for ensuring that firm employees are adequately supervised by the firm's certified individual.
(b) Within 10 business days of a change to the information submitted in the mitigation certification application, the certified individual shall submit to the Department a written and signed notification listing each change. The change will not take effect until the Department provides written approval of the change.
§ 240.114. Application filing deadline.
(a) A person who anticipates conducting radon mitigation services shall file a complete application for certification a minimum of 30 days prior to the anticipated starting date of mitigation activities.
(b) A certified individual renewal application postmarked after the previous certified individual certification expiration date will be charged a late application fee as set forth in Appendix A (relating to radon certification fee schedule).
CERTIFICATION FOR RADON LABORATORY § 240.121. Requirements for radon laboratory certification.
(a) A person in this Commonwealth or a person analyzing devices placed or retrieved in this Commonwealth may not perform laboratory analysis or represent or advertise that the person may perform laboratory analysis of radon testing devices supplied to the public or of samples or devices received from the public or from other certified persons, unless that person has first applied for and obtained radon laboratory analysis certification from the Department or is a firm employee of a certified laboratory firm.
(b) For a firm to perform radon laboratory analysis it shall employ at least one individual certified to perform laboratory analysis who is in responsible charge of the firm's laboratory radon analytical activities, and the firm shall submit an application for certification and receive certification from the Department.
§ 240.122. Prerequisites for radon laboratory certification.
(a) Individual certification for laboratory analysis. A person will not be certified to perform radon laboratory analysis unless the person has:
(1) Completed a Department-approved course on radon.
(2) Had 1 year professional experience in performing laboratory analysis of radon measurement devices or samples or is certified in Health Physics by the American Board of Health Physics, or equivalent certification or professional work experience, or both, as determined by the Department.
(3) Received a bachelors degree in the physical sciences or engineering or related fields as approved by the Department, or the education or professional work experience equivalent to a degree, as determined by the Department.
(4) Submitted a complete and accurate application to the Department, including applicable fees.
(b) Firm certification for laboratory analysis. If the applicant for radon laboratory certification is a firm, it shall employ at least one individual who is certified to perform radon laboratory analysis and who is in responsible charge of the laboratory radon analytical activities.
(1) If the firm loses its certified individual, all of the following apply:
(i) The firm owner shall notify the Department in writing within 5 business days of losing its certified individual.
(ii) The firm's certification automatically lapses and is void until the Department approves in writing the firm owner's written and signed request for a certified individual to be in responsible charge of that firm's radon laboratory activities.
(2) If a laboratory firm employee is no longer under the responsible charge of the firm's certified individual, the following apply:
(i) The firm's certified individual shall notify the Department within 10 business days of this change.
(ii) The firm employee's Department listing becomes invalid.
(3) Activities of the laboratory firm employee shall be conducted in accordance with the signed laboratory firm employee application.
(4) Each laboratory firm employee applicant shall submit all of the following:
(i) A completed and signed laboratory firm employee application as provided by the Department.
(ii) For firm employees hired after January 24, 2019, a document signed by the certified individual that the firm employee received initial training pursuant to subsection (b)(6).
(5) Each laboratory firm employee shall receive written approval from the Department prior to conducting radon laboratory activities as a laboratory firm employee.
(6) For firm employees hired after January 24, 2019, the firm's certified individual shall ensure that each firm employee receives initial training before participating in radon laboratory activities. Initial training may be given by the firm's certified individual or through a Department-approved training program. The firm's certified individual shall document that each firm employee has received initial training that includes, at a minimum, the following:
(i) General information regarding radon and the risks associated with radon exposure.
(ii) Information regarding radon laboratory analysis methods, protocols and standards.
(iii) Information regarding QA/QC for the laboratory device(s).
(iv) Information regarding necessary record keeping.
(7) The firm's certified individual shall ensure that each firm employee receives continuing education every two years. Continuing education may be given by the firm's certified individual or through a Department-approved training program. The firm's certified individual shall document that each firm employee has received continuing education. Continuing education records shall be retained for 5 years and include, at a minimum, the requirements set forth in subsection (b)(6)(ii)—(iv).
(c) Additional requirements. If the applicant for radon laboratory certification is a firm, or an individual performing laboratory analysis and not working for a certified laboratory, the applicant shall also have a QA program and a continuing education program as required under §§ 240.306 and 240.604 (relating to continuing education program; and QA requirements for testing using primary devices). In addition, the applicant shall be successfully enrolled in a Department-approved radon measurement proficiency program as required under § 240.307 (relating to radon measurement proficiency program).
§ 240.123. Radon laboratory application contents.
(a) An application for radon laboratory certification, by an individual or a firm, shall be submitted to the Department in writing on forms provided by the Department and must contain all of the following:
(1) Evidence that the applicant has the certification prerequisites contained in § 240.122 (relating to prerequisites for radon laboratory certification). The application must include the duties assigned to the certified individual in responsible charge of the laboratory analysis activities.
(2) A nonrefundable fee as set forth in Appendix A (relating to radon certification fee schedule).
(3) The applicant's name, address, and telephone number. It must also indicate if the applicant is an individual, partnership, limited partnership, corporation or other entity. The application must include, when appropriate, the name and address of every officer, general and limited partner, director, principal shareholder, parent corporation and certified person within the applicant's organization.
(4) Compliance information, including descriptions of notices of violation, administrative orders, civil penalty assessments and actions for violations of the act, this chapter or a term or condition of a certification.
(5) Other information the Department may require related to an applicant's qualifications or technical or administrative information related to laboratory analysis of radon samples.
(6) A verification by the applicant that the information contained in the application is correct to the best of the applicant's information and belief. This verification is subject to the penalties of 18 Pa.C.S. § 4904 (relating to unsworn falsification to authorities).
(7) If the applicant for laboratory certification is a firm, the application shall include a demonstration that the firm's certified individual will maintain adequate span of control over the firm's employees. This demonstration shall at least include:
(i) Information regarding the initial training and continuing education given to firm employees that is required by § 240.122(b)(6) and (b)(7) (relating to prerequisites for radon laboratory certification).
(ii) The firm's protocol for ensuring that firm employees are adequately supervised by the firm's certified individual.
(b) Within 10 business days of a change to the information submitted in the laboratory certification application, the laboratory certified individual shall submit to the Department a written and signed notification listing each change.
§ 240.124. Application filing deadline.
(a) A person who anticipates performing laboratory analysis of samples to determine radon concentrations shall file a complete application for laboratory analysis certification a minimum of 30 days prior to the anticipated starting date of laboratory analysis.
(b) A laboratory individual certification application postmarked after the previous laboratory individual certification expiration date will be charged a late application fee as set forth in Appendix A (relating to radon certification fee schedule).
[Continued on next Web Page]
No part of the information on this site may be reproduced for profit or sold for profit.This material has been drawn directly from the official Pennsylvania Bulletin full text database. Due to the limitations of HTML or differences in display capabilities of different browsers, this version may differ slightly from the official printed version.