PROPOSED RULEMAKING
ENVIRONMENTAL QUALITY BOARD
[25 PA. CODE CHS. 216, 218, 221, 223, 227
AND 228]
Radiological Health
[27 Pa.B. 5703] The Environmental Quality Board (Board) proposes to amend Chapters 216, 218, 221, 223, 227 and 228. The proposed amendments update the standards for the safe use of radiation-producing machines.
This proposal was adopted by the Board at its meeting of August 19, 1997.
A. Effective Date
These proposed amendments will be effective immediately upon publication in the Pennsylvania Bulletin as final rulemaking.
B. Contact Persons
For further information, the contact persons are Stuart R. Levin, Chief, Division of Radiation Control, Bureau of Radiation Protection, 13th Floor, Rachel Carson State Office Building, P. O. Box 8469, Harrisburg, PA 17105-8469, (717) 787-3720; and Marylou Barton, Assistant Counsel, Bureau of Regulatory Counsel, RCSOB, 9th Floor, 400 Market Street, P. O. Box 8464, Harrisburg, PA 17105-8464, (717) 787-7060.
C. Statutory Authority
These amendments are proposed under the authority of the following statutes:
Sections 301 and 302 of the Radiation Protection Act (act) (35 P. S. §§ 7110.301 and 7110.302), which, respectively, direct the Department to develop and conduct comprehensive programs for the registration, licensing, control, management, regulation and inspection of radiation sources and radiation source users, and delegates to the Board the power to adopt the regulations of the Department of Environmental Protection (Department) to implement the act.
Section 1920-A of The Administrative Code of 1929 (71 P. S. § 510-20), which authorizes and directs the Board to adopt regulations necessary for the proper performance of the work of the Department.
D. Background and Purpose
In 1987, the Board substantially updated its radiological health regulations to provide for compatibility with other states. These updates were published at 17 Pa.B. 5235 (December 19, 1987). Technological advances in the use of X-ray and accelerator equipment and the need to establish and maintain radiation protection standards at least as stringent as the Federal standards provide the basis for these revisions to the existing radiological health regulations.
The present regulations in these chapters were written in 1982-1983, prior to their effective date of December 19, 1987. In the meantime, certain advances have occurred, principally in the medical profession, which existing regulations do not address. These are new modalities for diagnosis and treatment now which did not exist when the existing regulations were being written and promulgated. Particle accelerators, particularly for use in medical applications, have undergone changes in design and function which were only beginning to emerge when the existing regulations were formulated.
The proposed amendments are based on the current Parts B, F, H and I of the 1995 version of the Suggested State Regulations for Control of Radiation (SSR) which was published by the Conference of Radiation Control Program Directors (CRCPD). Federal and State regulations for radiation sources and radiation source users are based on the SSR. Included in the SSR are amendments to Food and Drug Administration regulations.
The purpose of these proposed amendments is to bring existing regulations up-to-date by offering better protection to the employes and patients (for medical diagnosis and treatment applications) and to address health and safety concerns, including the reduction in unnecessary radiation exposure to patients and employes/operators. Department regional staff have encountered difficulties in adapting existing regulations to new technologies and modalities, especially in the diagnostic and therapeutic application of radiation in medicine, some of which relate not only to the machines and equipment used in generating ionizing radiation, but also in the safety of the personnel working with this equipment and in the safety of patients undergoing medical diagnosis and treatment. One of the goals of the Department is the reduction toward elimination of unnecessary radiation exposure, and the intent of the revisions in the regulations is to close some regulatory gaps and work toward achieving this goal.
As required by section 301(c)(14) of the act, the Department provided the Radiation Protection Advisory Committee (Committee) with an opportunity to review the proposed amendments and to advise the Department prior to submittal to the Board. The proposal was provided to the Committee for review on October 24, 1996, December 12, 1996, and March 20, 1997. The Committee provided oral and written comments at each meeting.
In response to the Committee members' comments, the Department revised the proposed amendments during the meetings.
E. Summary of Regulatory Requirements
The proposed amendments revise current radiation protection regulations to reflect the current technological advancements in radiation equipment design. A description of the proposed amendments is provided as follows:
Chapter 216. Registration of Radiation-Producing Machines.
Section 216.2(c)--(e) is proposed to be added to address the certificates of registration which are sent to registrants after they have properly completed their registration form and remitted the correct registration fee to the Department.
Section 216.4 is proposed to be renamed from ''Re- registration'' to ''Renewal of certificate of registration.'' Subsection (a) is proposed to be revised to allow the Department to combine the renewal and fee processes into one form. The Department and registrant would realize a cost and resource savings by eliminating one mailing.
A new § 216.4a is proposed to be added to clarify the requirements for terminating the use of X-ray equipment. A registrant will be required to: (1) terminate use of radiation-producing machines; (2) properly transfer or dispose of radiation-producing machines; (3) submit a record of disposal of each radiation-producing machine to the Department; (4) remit any outstanding registration fees owed to the Department; and (5) request termination of the certificate of registration in writing to the Department.
Chapter 218. Fees.
Section 218.1(b)(1) is proposed to be rewritten for clarification to state that the fee chapter applies to a person who is required to register a radiation-producing machine.
Section 218.11(b) is proposed to be revised to allow the Department to combine the registration renewal and the annual invoice into one form. The current system of separate forms for renewal of registration and an invoice is too cumbersome, expensive and time consuming for both the registrant and the Department.
Section 218.11(d) contains a proposed change of the payment time from 15 to 30 days to make it consistent with Chapter 216.
Chapter 221. X-Rays in the Healing Arts.
In general, the diagnostic X-ray sections of Chapter 221 are proposed to be rearranged and updated as necessary. The updated portions are based on the SSR. A new heading for CT machines is an expansion of the current § 221.62 (relating to computerized tomography).
In § 221.2 (relating to definitions), some definitions are proposed to be added and some deleted to maintain similarity with the SSR's. The added definitions are: ''AAPM,'' ''ACR,'' ''dental panoramic system,'' ''filtration,'' ''fluoroscopic system,'' ''intensifying screen,'' ''intraoral dental radiography,'' ''kV,'' ''kVp,'' ''licensed practitioner of the healing arts,'' ''mA,'' ''mAs,'' ''mR,'' ''mobile X-ray system,'' ''patient,'' ''peak tube potential,'' ''portable radiation system,'' ''positive beam limitation,'' ''protective barrier,'' ''qualified expert,'' ''registrant,'' ''SID,'' ''serial radiography'' and ''timer.''
Definitions that are proposed to be deleted are: ''assembler,'' ''attenuation block,'' ''beam monitoring system,'' ''cooling curve,'' ''filter,'' ''gonad shield,'' ''irradiation,'' ''kilowatt second (kWs)'' and ''source receptor distance (SID).''
Section 221.11 (relating to registrant responsibilities) is proposed to be amended as follows: subsection (a)(2) is proposed to be added to allow the Department to require registrants to comply with the Department of State's professional licensing requirements; subsection (a)(3) is proposed to be added to allow the Department to require registrants to comply with the Department of Health's requirements for auxiliary personnel using X-ray equipment; subsection (c) is proposed to be amended to include the information needed on the X-ray technique chart; subsection (k) is proposed to be added to require the use of compatible film and intensifying screen combinations; and, subsection (l) is proposed to be added to require a registrant to have a quality assurance program.
Section 221.15 (relating to use of X-rays in research on humans) contains new proposed regulations on the use of X-rays in research on humans. Registrants are exempted from the requirements of this section if the research is conducted, funded, supported or regulated by a Federal agency which has implemented the Federal policy for the protection of human subjects.
Section 221.21 (relating to diagnostic equipment requirements) is proposed to be updated to include the reference to 21 CFR 1020.33 (relating to computed tomography equipment).
Section 221.21, 221.28--221.30 and 221.32a--221.44a are derived from the current §§ 2221.22--221.56.
Sections 221.29 and 221.30 (relating to kilovoltage accuracy; and exposure reproducibility) are proposed to be added.
Sections 221.31--221.49 and 221.51--221.56, 221.61, 221.62, 221.71--221.76 and 221.81--221.102 are proposed to be deleted.
Proposed § 221.38a (relating to entrance exposure rate) is added to update the entrance exposure rates for fluoroscopes with and without a high level control. The new section is derived from current § 221.33 (relating to entrance exposure rate limits) has no limit when the high level control is activated. The proposed amendment will limit the output to 20 R per minute when the high level control is activated and also limit all image intensified fluoroscopes to 10 R per minute.
The current version of § 221.62 (relating to computerized tomography) is proposed to be replaced by a new heading, ''Computed Tomography X-ray Systems'' found in §§ 221.201--221.205. The current regulation pertaining to computerized tomography addresses only the location of the control panel, auxiliary support for the patient and visual indication of X-ray production at the control panel. The proposed amendments were developed to improve the quality of computed tomography (CT) imaging while minimizing the radiation dose to the patient. The proposed regulations are based on the SSR, 21 CFR 1020.33 (relating to computed tomography (CT) equipment) and the Department's CT study.
Proposed § 221.201 (relating to definitions) is a new section of definitions specifically for the CT regulation. The new definitions are: ''CT--computed tomography,'' ''CTDI--computed tomography dose index,'' ''CS--contrast scale,'' ''CT conditions of operation,'' ''CT number,'' ''elemental area,'' ''gantry,'' ''lux,'' ''MSAD--multiple scan average dose,'' ''multiple tomogram system,'' ''noise,'' ''nominal tomographic section thickness,'' ''performance phantom,'' ''picture element,'' ''pixel,'' ''reference plane,'' ''scan,'' ''scan increment,'' ''scan sequence,'' ''scan time,'' ''sensitivity profile,'' ''single tomogram system,'' ''technique factors,'' ''tomogram,'' ''tomographic plane'' and ''tomographic section.''
The entire heading containing requirements for therapeutic X-ray and electron beam systems with energies of 1 MeV and above was deleted because it was superseded by the new requirements in Chapter 228 (relating to radiation safety requirements for particle accelerators).
Proposed § 221.202 (relating to equipment requirements) includes subsections concerning termination of exposure, tomographic plane indication and alignment, status indicators and control switches, indication of CT conditions of operation, extraneous radiation, beam quality and additional requirements applicable to CT X-ray systems containing a gantry manufactured after September 3, 1985.
Proposed § 221.203 (relating to design requirements) contains requirements for oral communication between the patient and operator and viewing systems.
Proposed § 221.204 (relating to radiation measurements and performance evaluations) contains the requirements for radiation measurements and performance evaluations.
Proposed § 221.205 (relating to operating procedures) contains two subsections. Subsection (a) requires that certain information be available at the control panel such as instructions on using CT phantoms. Subsection (b) limits the use of the CT if measurements or performance evaluations exceed tolerances established by the facility's qualified expert.
Chapter 223. Veterinary Medicine.
Proposed § 223.7 (relating to structural shielding) is the current § 223.12 with the cross reference to § 219.21 (relating to radiation protection programs) corrected to § 219.51 (relating to radiation dose limits for individual members of the public).
Proposed § 223.8 (relating to operating procedures) has been renumbered from § 223.13 and clarifies the wording of the current § 223.13 but does not change the intent. Subsection (d) requires that all exposures be ordered by a veterinarian.
The heading of § 223.11 is proposed to be amended to read ''Radiographic equipment.''
Section 223.11(a)(2) is proposed to be added so that the veterinarians will not have to refer to Chapter 221 (relating to X-rays in the healing arts).
Section 223.11(b)(2) and (3) were poorly written and were vague about what was required. These proposed amendments clarify the requirement for proper collimation.
The proposed amendments to § 223.11(d)(1) and (2) expand on timer requirements and require X-ray timers to be accurate.
The proposed amendment to § 223.11(e) adds a new requirement taken in part from the SSR. It requires that the X-ray output from an X-ray unit be consistent. This will prevent repeat exposures due to X-ray equipment with an inconsistent output.
The proposed amendment to § 223.11(f) states a tube stand is now only required when it would not interfere with the procedure. This would allow the veterinarian to hold the tube head when this would be more efficient and when the X-ray unit would be in danger of destruction from the moving and kicking by large animals.
Proposed § 223.11(g) is from the SSR and is also found in the regulations of other states. This requirement is met by newer X-ray equipment.
The proposed § 223.12a is a modification of the current § 223.11(g) fluoroscopic equipment requirements. This modification is a relaxation of the current regulations since it exempts veterinary fluoroscopes from the entrance exposure rate requirements and addresses only the health and safety of the operators.
A new proposed § 223.13a (relating to therapeutic systems) is a modification of the current § 223.11(g) (equipment used for therapeutic purposes). This is a relaxation of the current regulations, since it exempts the therapeutic systems from calibration and spot check requirements.
Chapter 227. Radiation Safety Requirements for Analytical X-Ray Equipment, X-Ray Gauging Equipment and Electron Microscopes.
Chapter 227 is proposed to be rearranged for clarity and some of the wording was also modified. Current §§ 227.11 and 227.12 were combined into the proposed §§ 227.11a and 221.12a (relating to equipment requirements; and area requirements). Current § 227.13 became the proposed § 227.13a (relating to operating requirements).
Proposed § 227.11a(h) has been added to provide regulation for vacuum spectroscopy. Vacuum spectrographs will be exempted from §§ 227.12a and 227.13a, but shall meet the requirements of § 227.14 (relating to personnel procedures).
Proposed § 227.13a(c) and (d) are additions to the current regulations to provide compatibility with the SSR.
Chapter 228. Radiation Safety Requirements for Particle Accelerators.
Definitions are proposed to be added to § 228.2 (relating to definitions) as a result of the expansion of the regulations concerning accelerators in the healing arts. The additional definitions are: ''applicator,'' ''beam-limiting device,'' ''beam scattering filter,'' ''central axis of the beam,'' ''dose monitoring system,'' ''dose monitor unit,'' ''existing equipment,'' ''field flattening filter,'' ''field size,'' ''filter,'' ''isocenter,'' ''leakage radiation,'' ''moving beam therapy,'' ''new equipment,'' ''normal treatment distance,'' ''phantom,'' ''primary dose monitoring system,'' ''qualified expert,'' ''radiation detector,'' ''radiation head,'' ''secondary dose monitoring system,'' ''shadow tray,'' ''spot check,'' ''stationary beam therapy,'' ''subsystem,'' ''target,'' ''tube housing assembly,'' ''useful beam'' and ''wedge filter.''
A proposed new heading titled, ''administrative controls,'' was adapted from §§ 221.11 and 221.12 and placed in this chapter.
A proposed new heading titled, ''notification and licensing procedures,'' was added to allow the Department to license particle accelerators. Sections 228.21a, 228.22a, 228.23a, 228.24a, 228.25a and 228.26a were adapted from §§ 217.51--217.57 (relating to specific licenses-general conditions) which relate to the licensing of radioactive material.
The current heading, ''General Radiation Safety Requirements,'' is proposed to be renumbered from §§ 228.21--228.26 to §§ 228.31a, 228.32a, 228.33a and 228.34a--228.39. A new § 228.36(c) is proposed to be added to inform the licensee or registrant what is required for and exempted from the calibration of an independent radiation monitor. Proposed § 228.39 (relating to records) is added to clarify the recordkeeping requirements.
The heading, ''Radiation Safety Requirements for Industrial and Research Accelerators'' is proposed to be renumbered from §§ 228.31--228.34 to §§ 228.41a, 228.42, 228.43 and 228.44. Proposed § 228.45 (relating to portable or mobile accelerators) is added to cover portable and mobile particle accelerators.
The proposed heading, ''Radiation Safety Requirements for Accelerators Used in the Healing Arts,'' is expanded by adapting the appropriate sections from Chapter 221, titled, Therapeutic X-ray and Electron Beam Systems With Energies of 1 Mev And Above.'' These proposed sections are numbered §§ 228.61--228.76. Section 221.41 is proposed to be deleted.
F. Benefits, Costs and Compliance
Executive Order 1996-1 requires a cost/benefit analysis of the proposed amendments.
Benefits
As set forth in this proposal, users of radiation-producing machines will be required to comply with radiation protection standards that will not only protect operators of the machines but will also protect the general public.
Compliance Costs
Compliance costs are expected to be minimal. The Department has been implementing many of the proposed requirements by recommendation. No financial assistance is believed to be necessary.
Compliance Assistance Plan
Compliance assistance is available to existing holders of a registration of radiation-producing machines and equipment. These range from small X-ray facilities such as dentists, podiatrists, veterinarians, and the like, to large institutions such as colleges and universities, medical centers and industrial complexes, all of which the Department presently regulates and inspects. The Department will issue technical guidance to registrants as recommended by the Committee.
Paperwork Requirements
The proposed amendments will not significantly change paperwork requirements.
G. Sunset Review
These regulations will be reviewed in accordance with the sunset review schedule published by the Department to determine whether the regulations effectively fulfill the goals for which they were intended.
H. Regulatory Review Act
Under section 5(a) of the Regulatory Review Act (71 P. S. § 745.5(a)), on October 16, 1997, the Department submitted a copy of the proposed amendments to the Independent Regulatory Review Commission (IRRC) and the Chairpersons of the Senate and House Environmental Resources and Energy Committees. In addition to submitting the proposed amendments, the Department has provided IRRC and the Committees with a copy of a detailed Regulatory Analysis Form prepared by the Department. A copy of this material is available to the public upon request.
If IRRC has objections to any portion of the proposed amendments, it will notify the Department within 10 days of the close of the Committees' comment period. The notification shall specify the regulatory review criteria which have not been met by that portion. The Regulatory Review Act specifies detailed procedures for review, by the Department, the Governor and the General Assembly before final publication of the regulations.
I. Public Comments
Written comments--Interested persons are invited to submit comments, suggestions or objections regarding the proposed amendments to the Environmental Quality Board, P. O. Box 8477, Harrisburg, PA 17105-8477 (express mail: Rachel Carson State Office Building, 15th Floor, 400 Market Street, Harrisburg, PA 17101-2301). Comments submitted by facsimile will not be accepted. Comments, suggestions or objections must be received by the Board by December 30, 1997. Interested persons may also submit a summary of their comments to the Board. The summary may not exceed one page in length and must be received by December 30, 1997. The one-page summary will be provided to each member of the Board in the agenda packet distributed prior to the meeting at which the final-form regulations will be considered.
Electronic comments--Comments may be submitted electronically to the Board at RegComments@A1.dep. state.pa.us and must also be received by the Board by December 30, 1997. A subject heading of the proposal and a return name and address must be included in each transmission. If an acknowledgment of electronic comments is not received by the sender within 2 working days, the comments should be retransmitted to ensure receipt.
JAMES M. SEIF,
ChairpersonFiscal Note: 7-329. No fiscal impact; (8) recommends adoption.
Annex A
TITLE 25. ENVIRONMENTAL PROTECTION
PART I. DEPARTMENT OF ENVIRONMENTAL PROTECTION
Subpart D. ENVIRONMENTAL HEALTH AND SAFETY
ARTICLE V. RADIOLOGICAL HEALTH
CHAPTER 216. REGISTRATION OF RADIATION-PRODUCING MACHINES § 216.2. Registration.
* * * * * (c) A certificate of registration will be issued to a person whose registration becomes valid under subsection (b).
(d) A registrant shall have the currently valid certificate of registration available for inspection by the Department.
(e) A certificate of registration issued under this chapter may not be transferred, assigned or in any manner disposed of, either voluntarily or involuntarily, to any person without the written approval of the Department.
§ 216.4. [Re-registration] Renewal of certificate of registration
(a) The Department will send [a] an application for renewal [form] of the certificate of registration to the registrant at least 2 months prior to the expiration date [of the existing registration] on the certificate of registration. The application for renewal will include references to the fee due under § 218.11 (relating to annual registration and license fees). [The registrant shall return the completed renewal form within 30 days after receipt of the renewal form.]
(b) [The renewal becomes valid upon receipt of the properly completed form and the fee required under Chapter 218 (relating to fees)] An applicant for renewal of a registration shall submit a signed application and the fee required under § 218.11 prior to the expiration date of the certificate of registration.
(c) The renewal becomes valid upon receipt of the properly completed application and the fee required under Chapter 218 (relating to fees).
§ 216.4a. Expiration and termination of certificates of registration.
(a) A certificate of registration expires on the date specified on the certificate of registration. Expiration of the certificate of registration does not relieve the registrant from the requirements of this article.
(b) When a registrant decides to terminate all activities involving radiation-producing machines under the certificate of registration, the registrant shall notify the Department immediately, in writing, and request termination of the certificate of registration. This notification and request for termination of the certificate of registration shall be in accordance with subsection (c).
(c) If a registrant does not submit a renewal for a certificate of registration under § 216.4 (relating to renewal of certificate of registration), the registrant shall, on or before the expiration date specified in the certificate of registration, do the following:
(1) Terminate use of all radiation-producing machines.
(2) Properly transfer or dispose of all radiation-producing machines.
(3) Submit a record of disposal of each radiation-producing machine to the Department.
(4) Remit any outstanding registration fees owed to the Department.
(5) Request termination of the certificate of registration in writing to the Department.
CHAPTER 218. FEES
GENERAL § 218.1. Purpose and scope.
* * * * * (b) Except as otherwise specifically provided, this chapter applies to a person who:
(1) [Has filed a registration] Is required to register or renew registration for radiation-producing machines [required] under Chapter 216 (relating to registration of radiation-producing machines).
* * * * *
PAYMENT OF FEES § 218.11. Annual registration and license fees.
* * * * * (b) [Upon receipt of a] A registrant filing an initial registration under § 216.2 (relating to registration) or [re-registration form, the Department will issue] an application for renewal of a certificate of registration under § 216.4 (relating to renewal of certificate of registration) shall remit the appropriate fee [invoice] calculated by using the [registered] information on the registration or application form and the fee schedule in subsection (a). Fees for any initial registration under § 216.2 are payable [within 15 days after receipt of a fee invoice] upon the filing of the registration. Fees for the renewal of a certificate of registration are payable upon the submission of an application for a renewal of a certificate of registration. If the number of tubes increases after [a] an initial registration or [re-registration form] after an application for renewal has been filed with the Department, no additional fee is required until the time of the next registration. Likewise, if the number of tubes decreases during the year, no refund will be made for that year.
* * * * * (d) An initial application for a license shall be accompanied by a check payable to the Department [of Environmental Resources] in accordance with the fee schedule in subsection (c). Thereafter, the Department will issue an annual fee invoice based on the fee schedule in subsection (c). Fees are payable within [15] 30 days after receipt of a fee invoice.
* * * * *
CHAPTER 221. X-RAYS IN THE HEALING ARTS
GENERAL § 221.2. Definitions.
[As used in this chapter, the] The following words and terms, when used in this chapter, have the following meanings, unless, the context clearly indicates otherwise:
AAPM--American Association of Physicists in Medicine.
ACR--American College of Radiology.
* * * * * [Assembler--A person who assembles, replaces or installs one or more components into an x-ray system or subsystem.
Attenuation block--A block or stack of type 1100 aluminum alloy--the nominal chemical composition of type 1100 aluminum alloy is 99% minimum aluminum, .12% copper--or other materials having equivalent attenuation, having a thickness of 3.8 centimeters and a width and a length not less than 15 centimeters.]
* * * * * [Beam monitoring system--A system designed to detect and measure the radiation present in the useful beam.]
* * * * * [Cooling curve--The graphical relationship between heat units stored and cooling time.]
* * * * * Dental panoramic system--A device intended to produce a radiographic image of the entire dental arch on one film.
* * * * * [Filter] Filtration--* * *
* * * * * Fluoroscopic system--(See fluoroscopic imaging assembly).
* * * * * [General purpose radiographic x-ray system--A radiographic x-ray system which, by design, is not limited to radiographic examination of specific anatomical regions.
Gonad shield] Gonads--[A protective barrier for] The testes or ovaries.
* * * * * Intensifying screen--A fluorescent screen which transforms incident X-ray photons into a visible image.
Intraoral dental radiography--A modality of dental radiography in which the image receptor is placed inside a patient's oral cavity.
[Irradiation--The exposure of matter to ionizing radiation.]
* * * * * kV--Kilovolts
kVp--Peak tube potential (See Kilovolts peak).
* * * * * [Kilowatt second (kWs)--The term is equivalent to 103. kV. mA s, or
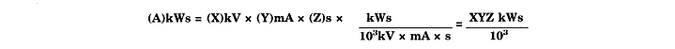
* * * * * Licensed practitioner of the healing arts--An individual licensed by the Commonwealth to practice the healing arts, which for the purposes of this article shall be limited to medicine, surgery, dentistry, osteopathy, podiatry and chiropractic.
* * * * * mA--Milliampere
mAs--Milliampere second
mR--Milliroentgen
* * * * * Mobile X-ray system--(See X-ray equipment)
Patient--An individual subjected to healing arts examination, diagnosis or treatment.
Peak tube potential--The maximum value of the potential difference across the X-ray tube during an exposure.
* * * * * Portable radiation system--(See X-ray equipment)
* * * * * Positive beam limitation--The automatic or semiautomatic adjustment of an X-ray beam to the size of the selected image receptor, whereby an X-ray exposure cannot be made without an adjustment.
* * * * * Protective barrier--A barrier of radiation absorbing material used to reduce radiation exposure. The term includes the following types:
(i) Primary protective barrier--Material used to reduce radiation exposure from the useful beam.
(ii) Secondary protective barrier--Material used to reduce exposure from stray, leaked or scattered radiation.
* * * * * Qualified expert--An individual having the knowledge and training to measure ionizing radiation, to evaluate safety techniques and to advise regarding radiation protection needs--for example, individuals certified in the appropriate field by the American Board of Radiology, the American Board of Health Physics or the American Board of Medical Physics, or those having equivalent qualifications. With reference to the calibration or radiation therapy equipment, an individual having, in addition to these qualifications, training and experience in the clinical applications of radiation physics to radiation therapy--for example, individuals certi-fied in therapeutic radiological physics or X-ray and radium physics by the American Board of Radiology, Radiation Oncology Physics by the American Board of Medical Physics or those having equivalent qualifications.
* * * * * Registrant--A person who is legally obligated to register with the Department under this article and the act.
* * * * * SID--Source-image receptor distance--The distance from the source to the center of the input surface of the image receptor.
* * * * * Serial radiography--Radiographic images produced in regular sequence.
* * * * * [Source-image receptor distance (SID)--The distance from the source to the center of the input surface of the image receptor.]
* * * * * Technique factors--[The conditions of operation specified as follows] The following conditions of operation:
* * * * * (ii) For field emission equipment rated for pulsed operation, peak tube potential in kV, [and] number of [x-ray] X-ray pulses and either tube current or product of tube current and time.
* * * * * Therapeutic [x-ray or electron] X-ray system--* * *
Timer--An electronic device which is capable of measuring an X-ray exposure.
* * * * * X-ray equipment--An [x-ray] X-ray system, subsystem or component thereof. Types of [x-ray] X-ray equipment are as follows:
* * * * * (iii) Stationary [x-ray] X-ray equipment--X-ray equipment which is installed in a fixed location or vehicle.
* * * * *
ADMINISTRATIVE CONTROLS § 221.11. Registrant responsibilities.
(a) [No person may operate or permit the operation of radiation-producing machines unless the machines and installation meet the applicable requirements of this article.] The registrant is responsible for directing the operation of X-ray systems under his administrative control and shall do the following:
(1) Assure that the requirements of this article are met in the operation of the X-ray systems.
(2) Permit only auxiliary personnel who have met the applicable requirements of 49 Pa. Code Part I, Subpart A (relating to professional and occupational affairs) to operate X-ray systems for diagnostic or therapeutic purposes.
(3) Permit only auxiliary personnel employed by a health care facility regulated by the Department of Health, the Department of Public Welfare or the Federal government to operate X-ray systems for diagnostic or therapeutic purposes in accordance with written job descriptions and employe qualifications.
(b) An individual who operates [the x-ray] an X-ray [systems] system shall be [adequately] instructed adequately in the safe operating procedures and be competent in the safe use of the equipment. The instructions shall include, but not be limited to, items included in Appendix A (relating to determination of competence).
(c) A chart, which specifies the techniques for examinations performed with the system, shall be provided in the vicinity of [the] each diagnostic [x-ray] X-ray system's control panel. This chart shall include information pertinent to the particular examination, such as:
(1) The patient's body part and anatomical size, or body part thickness, or age (for pediatrics), versus technique factors to be utilized.
(2) The type and size of the film or film-screen combination.
(3) The type of grid, if any.
(4) The type and location of placement of patient shielding--for example, gonad, and the like.
(5) For mammography, indication of kVp/target/filter combination.
* * * * * (e) Except for patients who cannot be moved out of the room, only the staff and ancillary personnel required for the medical procedure or training shall be in the room during the radiographic exposure. The following apply for individuals other than the patient being examined:
(1) Individuals shall be positioned so that no part of the body will be struck by the useful beam unless protected by at least 0.5 millimeter lead equivalent [shielding] material. The lead equivalent material is to be determined at 60 kV.
(2) [Staff and ancillary personnel] All persons required for the medical procedure shall be protected from the scatter radiation by protective aprons or whole protective barriers of at least 0.25 millimeter lead equivalent.
(3) [Other patients not being radiographed] A patient who cannot be removed from the room shall be protected from the scatter radiation by protective barriers of at least 0.25 millimeter lead equivalent material or shall be so positioned that the patient is not in the direct line of the useful beam and the nearest portion of the body is at least 2 meters from both the tube head and the nearest edge of the image receptor.
(f) During diagnostic procedures in which the gonads are in the useful beam, gonad shielding of [not less than .25] at least 0.5 millimeter lead equivalent shall be used for patients except for cases in which this would interfere with the diagnostic procedure.
(g) An individual may not be exposed to the useful beam except for healing arts purposes or under § 221.15 (relating to use of X-rays in research on humans). An exposure shall be authorized by a licensed practitioner of the healing arts. This provision specifically prohibits deliberate exposure for the following purposes:
(1) Exposure of an individual for training, demonstration or other nonhealing arts purposes [unless there are also healing arts requirements and proper prescription has been provided].
(2) Exposure of an individual for the purpose of healing arts screening except as [approved] authorized by the Department. When requesting [approved] authorization, the registrant shall submit the information as outlined in § 221.13 (relating to information to be submitted by persons proposing to conduct healing arts screening). [If information submitted to the Department becomes invalid or outdated, the registrant shall immediately notify the Department.]
(h) If a patient or film requires auxiliary support during a radiation exposure the following apply:
* * * * * (3) [No] An individual may not be used routinely to hold film or patients.
(4) For intraoral dental radiography, neither the tube housing nor the cone shall be held during an exposure.
* * * * * (k) The screen and film system used shall be spectrally compatible and evaluated with respect to screen condition to assure proper system speed. Film cassettes without intensifying screens may not be used for any routine diagnostic radiological imaging, with the exception of veterinary radiography and standard intraoral dental radiography film packets.
(l) The registrant shall have a quality assurance program. This quality assurance program shall be in accordance with guidelines promulgated by the ACR, the AAPM or another accredited organization.
§ 221.12. [Information and maintenance record] Records, maintenance and associated information.
* * * * * § 221.13. Information to be submitted by persons proposing to conduct healing arts screening.
[Continued on next Web Page]
No part of the information on this site may be reproduced for profit or sold for profit.This material has been drawn directly from the official Pennsylvania Bulletin full text database. Due to the limitations of HTML or differences in display capabilities of different browsers, this version may differ slightly from the official printed version.