§ 401.11. Magnetic resonance Certificate of Need—statement of policy.
(a) Status and trends. Magnetic resonance (MR) is a category of service that uses the magnetic spin property of certain atomic nuclei to visualize and analyze tissue. Diagnostic techniques include both MR imaging and spectroscopy.
(1) MR imaging has proven to be a useful tool in the diagnosis of the following:
(i) Brain, brain stem, spinal cord disorders and demylenating diseases.
(ii) Diffuse or infiltrating diseases of the liver and kidney.
(iii) Early changes in ischemia and infarction of heart tissue.
(2) Ovarian and uterine tissue can be imaged well through MR. Also, the process can be used to evaluate growth and development of a fetus.
(3) MR spectroscopy has potential application for in vivo analysis of biochemical processes in healthy and diseased tissue. Potential applications include the following:
(i) Determination of early chemical changes in evolving cerebral or myocardial infarctions.
(ii) Determination of the chemical nature and specific diagnosis of tumors throughout the body.
(iii) Analysis of chemical parameters and changes in metabolic diseases of the liver and kidney.
(iv) In vivo analysis of early changes in multiple sclerosis.
(v) In vivo analysis of dementia-producing disorders such as Alzheimer’s disease.
(4) The Federal Food and Drug Administration (FDA) has approved MR imaging devices produced by certain manufacturers.
(5) On July 1, 1985, Blue Cross of Western Pennsylvania began coverage for all MR imaging scans performed on FDA-approved equipment provided that a certificate of need has been issued or a project has been deemed nonreviewable. Pennsylvania Blue Shield followed suit in October 1985. In November 1985, The Health Care Finance Administration announced that Medicare will provide reimbursement for MR imaging procedures for a limited number of diagnoses. As of April 1987, third party payers were not reimbursing facilities for spectroscopic services which are regarded as experimental.
(6) MR devices are available with one of three broad categories of magnets. Each type of magnet can be obtained in a variety of field strengths up to 2.5 Telsa. Higher field strength becomes important in MR spectroscopy. The three categories of magnets are:
(i) Resistive.
(ii) Permanent.
(iii) Superconductive.
(7) A recent development is the mobile MR unit. Equipment manufacturers predict that up to 40% of their market will be mobile units.
(8) A type of movable unit is the transportable and relocatable system. These are larger than mobile units and are not intended to be moved more than several times per year.
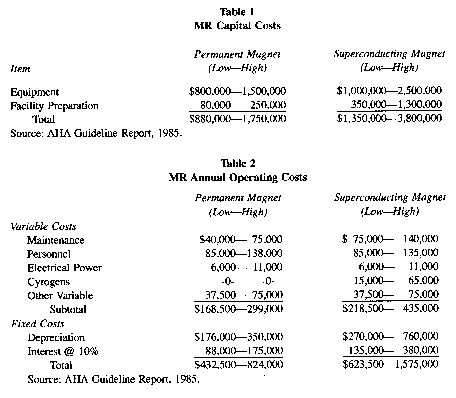
(9) Tables 1 and 2 show the estimated range of purchase and installation costs, and annual operating costs, respectively, as compiled by the American Hospital Association (AHA). Mobile systems will have the following additional costs:
(i) For the trailer—$500,000.
(ii) For the tractor—$50,000 to $125,000.
(10) Although MR imaging has distinct advantages over the less expensive computerized axial tomography (CAT) scan, there are important limitations. Seriously ill patients in monitored beds cannot be imaged because the presence of strong magnetic fields will affect electronic monitoring devices. Also, MR is contraindicated for patients with intravenous (IV) needles and ferrous metal implants. The AHA projects that across all disease categories, MR imaging will replace only about 34% of all CAT scans.
(11) Table 4, set forth in subsection (c), shows the present distribution of certificate of need approved MR devices in this Commonwealth.
(b) Policy. The following criteria will be used in reviewing certificate of need proposals related to magnetic resonance:
(1) The formula set forth in subsection (c) will be used in certificate of need reviews for MR imaging project proposals.
(2) To the extent feasible, and consistent with other criteria, MR devices should be dispersed throughout a region. Magnetic resonance imaging (MRI) services should be located so that 90% of the population of the region is within 1 hour travel time to a service.
(3) Applicants shall establish and document a policy that MR services will be provided regardless of a patient’s ability to pay.
(4) Shared arrangements may be any of the following:
(i) Reciprocal agreements among hospitals with CAT and MRI capability.
(ii) Reciprocal agreements involving use of a device located at a free-standing imaging center.
(iii) Reciprocal agreements involving use of a mobile or a transportable/relocatable unit.
(5) The Department will deem the needs of the population to be best served by hospitals proposing shared arrangements with other health care facilities.
(6) The Department will deem a shared arrangement to be economically more feasible than a single facility use proposal. When comparing two or more shared arrangement proposals, the Department will evaluate the following:
(i) The respective capabilities of the project applicants and associated facilities of each.
(ii) The anticipated volume of care of each.
(iii) The availability of reasonable start-up capital of each.
(iv) Projected marginal revenues of each.
(v) Projected marginal costs, variable costs and fixed costs of each.
(7) An applicant facility shall establish and document a policy that the hospitals in a region to be served by an MR unit will have equal access to the unit. Equal access will be characterized by the following:
(i) A scheduling priority based on patient need. Documented urgent or emergent cases will be given priority access.
(ii) A nondiscriminatory charge schedule.
(iii) Interhospital transport services with appropriate medical supervision established either directly by the applicant or through a mutually agreed upon arrangement with the referring facility.
(8) Charges for MRI services should be reasonably related to service cost. Charges should not exceed the median charge of Commonwealth providers of comparable MRI services by more than 20% without reasonable justification.
(9) Facilities providing MR services shall have a utilization review program that includes MR examinations.
(10) Hospitals offering MR shall be able to directly provide related diagnostic modalities such as the following:
(i) CAT full-body scanning.
(ii) Ultrasound.
(iii) Radionuclide scanning.
(iv) Conventional X-ray, including, but not limited to, arteriography.
(11) A board-certified or board-qualified physician trained in MRI shall be responsible for the operation of the MR facility and interpretation of the MR data. This work shall be the full-time activity of that physician or other physicians delegated by that physician if they have been trained in MRI.
(12) Medical physicist involvement is necessary for quality assurance, computer maintenance and training of staff in magnetic field theory and related issues.
(13) The MR program staff shall include the specialty of radiology and subspecialists appropriate to the applications intended, including experience in computed tomography or nuclear medicine.
(14) Facilities proposing to provide MR services shall also directly provide a variety of medical subspecialty services which include, but are not limited to, oncology, neurology, internal medicine, pathology and radiology.
(15) At least one staff person trained in cardio-pulmonary resuscitation (CPR) shall be on duty in the unit at all times.
(16) The facility shall have a program on image quality control of MR services and a program to calibrate and maintain its diagnostic equipment.
(17) The MR unit shall meet the standards recommended by the FDA.
(18) The area housing the MR unit shall be constructed in accordance with standards established by the manufacturer and Federal or State standards, or both, as may be developed.
(c) MR need.
(1) Several methodologies for projection of MRI utilization have been studied.
(i) The methodology used in New York State assumes a ratio of one MR unit per three fully utilized CAT scanners. New York considers 3,000 images per year as full utilization of an MR unit.
(ii) Massachusetts uses the AHA utilization model, at least in part.
(iii) Illinois will approve an MR unit at a hospital which does 4,500 CAT scans per year or more.
(iv) The Health Systems Agency of Southwestern Pennsylvania uses the methodology developed by the AHA.
(v) The AHA methodology is based on the opinions of an expert panel of physicians. The panel determined the percent of patients within discrete ICD-9-CM categories who would require MRI.
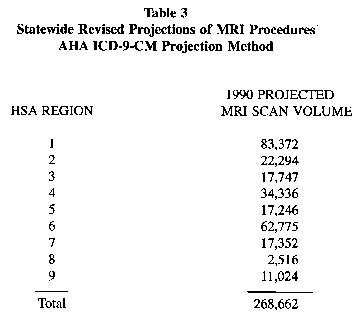
(2) The Department adopts the AHA methodology, as set forth in the AHA Hospital Technology Series, Vol. 11, No. 8, ‘‘NMR—Nuclear Magnetic Resonance Guideline Report’’ of 1983, to predict the number of initial and followup scans. The Statewide results of this methodology are given in Table 3.
(3) Throughput is the number of patients imaged per year. The Health Systems Agency of Southwestern Pennsylvania estimated that a unit is capable of 2,000 procedures per year. By the end of 1986, the 14 test sites in New York State were achieving an average throughput of 2,500 patients per year. Full utilization in New York State is 3,000. A radiological team headed by W. G. Bradley reports in ‘‘MR Installation, 18 Months Clinical Experience’’ that patient throughput at the MR operated by the 625-bed Huntington Medical Research Institute averages more than 12 patients per day with as many as 18 patients per day often being examined. By reaching reasonable throughput, the Huntington Institute has been able to keep average MR charges at about 25% higher than CAT. Twelve patients per day yields a total annual throughput of 3,000 patients.
(4) The following formula is used to predict the number of MRI devices needed in Pennsylvania.

(5) The Department of Health adopts 2,500 patients per year as a reasonable throughput for a single unit. Therefore, using the formula in paragraph (4), approximately 107 MR units will be adequate to meet the needs of patients in this Commonwealth.
(6) Table 4 shows the projected need for MR units by health service area. Rounding of fractional units next higher integer results in a total State need of 110 units.
Source The provisions of this § 401.11 adopted July 10, 1987, effective July 11, 1987, 17 Pa.B. 2946; amended August 5, 1988, effective August 6, 1988, 18 Pa.B. 3467; amended December 8, 1989, effective December 19, 1989, 19 Pa.B. 5222. Immediately preceding text appears at serial pages (127049) to (127052) and (129161) to (129163).
No part of the information on this site may be reproduced for profit or sold for profit.
This material has been drawn directly from the official Pennsylvania Code full text database. Due to the limitations of HTML or differences in display capabilities of different browsers, this version may differ slightly from the official printed version.