§ 401.16. Positron Emission Tomography (PET) services; interim criteria for Certificate of Need (CON) review—statement of policy.
(a) Acquisition of a PET scanner is subject to review under section 701 of the act (35 P. S. § 448.701).
(b) Certificate of Need review for proposed PET scanners will be conducted using the need methodology in subsection (c), and the review criteria in subsection (h).
(c) The Department will use the following methodology to determine the need for PET scanners in this Commonwealth:
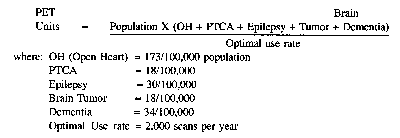
(d) The incidence rate for open heart surgery is derived from the formula found at Chapter 26 of the SHP as amended July 16, 1991.
(e) Incidence rates for percutaneous transluminal coronary angioplasty (PTCA), epilepsy, brain tumor and dementia are derived from a methodology developed by the American Hospital Association. This methodology determined which ICD-9 diagnostic codes involve conditions which might require a PET scan, the percentage of patients with the condition who would receive a scan and the average number of scans each patient would be likely to receive. Incidence data are based on 1989 hospital discharge data reported to the National Center for Health Statistics.
(f) The optimal use rate, expressed as number of scans per unit, is taken from a number of studies as well as from literature supplied to the Department by manufacturers of PET scan devices. This material supports a rate range of six to ten scans per device per day. A midpoint of eight scans per device per day is adopted, and results in a total annual use rate of 2,000 scans per device per year.
(g) Application of the formula in subsection (c) projects the following need by health planning area through the year 1995.
Health Planning Area Projected Need I 5 II 1 III 1 IV 2 V 1 VI 4 VII 1 VIII 0 IX 1 Total 16 (h) The Department will use the following review criteria in addition to the need projections in subsection (g) to determine the need for a PET service:
(1) A site where PET services are proposed to be offered shall be a site where the following clinical services also are currently offered:
(i) An open heart surgery program that is approved by the Department and where an average of at least 700 open heart cases per year during the most recent 3-year period were performed. In the interest of geographic distribution of PET services, the Department may waive this requirement in its consideration of an application from a hospital where at least 450 open heart cases per year were performed during the most recent 3-year period, if no other hospital in the same health planning area met the 700 case standard. The Department may also waive this requirement in its consideration of an application from a medical college.
(ii) A therapeutic cardiac catheterization service that is approved by the Department and that includes a PTCA program.
(iii) A full range of full-time, onsite related diagnostic modalities including conventional x-ray, full-body computed tomography, ultrasound, magnetic resonance imaging and other radio-nuclide scanning.
(2) The Department will consider applications for shared PET services. The standards in paragraph (1) shall be met by each hospital participating in the shared PET service.
(3) Hospitals in a region served by one or more PET scanners will have equal access to at least one of the units. Equal access will be characterized by the following:
(i) A scheduling priority based on patient need.
(ii) Services provided to patients from referring hospitals will be charged for the PET services at the same rate as patients in the hospital housing the PET scanner.
(iii) Transportation services with appropriate supervision established either directly through the sponsor or through a mutually agreed upon arrangement with the referral facilities.
(4) The equipment shall be certified for clinical use by the Federal Food and Drug Administration (FDA). The facility shall also present evidence of approval by the FDA of its New Drug Application (NDA) for the production of radiopharmaceuticals as part of the CON application.
(5) A PET service shall be under the medical direction of a physician who is board certified in nuclear medicine or nuclear radiology, or trained and licensed in nuclear cardiology and has additional documented experience and training in PET technology, including radiochemistry. The physician shall be licensed by the Nuclear Regulatory Commission to possess radiopharmaceuticals and perform diagnostic procedures employing radiopharmaceuticals in human beings.
(6) Additional staff for a PET service shall include at a minimum the following staff as appropriate:
(i) A radiochemist trained at the master’s or Ph.D. level in radiochemistry or radiopharmacy who also has a background in PET physics or radiochemistry and experience in radiopharmaceutical production.
(ii) A nuclear medicine technologist with training onsite or offsite in cyclotron operation and radiopharmaceutical production, and who will work under direction and supervision of the medical director.
(iii) Two radiological technologists with documented training in radiology, nuclear medicine or MRI/CT scanning and who are able to provide support in the areas of PET imaging systems operation, patient preparation for PET studies and image analysis and processing.
(7) The PET service should be available for operation at least 8 hours per day, 5 days per week. Evening and weekend hours are encouraged as an aid to accessibility.
(8) It is the policy of the Department to encourage efficient use of expensive technology through sharing of cyclotron facilities.
(9) Proposals to convert an existing research scanner to clinical use will be subject to CON review.
(10) Additional scanners in a health planning area beyond the need projected in subsection (g) will not be approved until previously approved PET scanners in the health planning area are operating at an average of 2,000 scans per year.
(i) This section shall serve as an interim policy until replaced with standards and criteria in the State Health Services Plan.
Source The provisions of this § 401.16 adopted May 21, 1993, effective May 22, 1993, 23 Pa.B. 2449.
No part of the information on this site may be reproduced for profit or sold for profit.
This material has been drawn directly from the official Pennsylvania Code full text database. Due to the limitations of HTML or differences in display capabilities of different browsers, this version may differ slightly from the official printed version.